– Total revenues of $1,044 million in the second quarter (Q2'24) (+9% Y/Y)
– Jakafi® (ruxolitinib) net product revenues of $706 million in Q2'24 (+3% Y/Y), total patients increased +7% Y/Y; raising the bottom end of full year 2024 guidance to a new range of $2,710 - $2,750 million
– Opzelura® (ruxolitinib) net product revenues of $122 million in Q2'24 (+52% Y/Y); continued uptake in atopic dermatitis (AD) and vitiligo in the U.S.; launch momentum and reimbursement expansion in vitiligo in Europe
– Incyte announces increased R&D focus on innovative high impact clinical programs; acquisition of Escient Pharmaceuticals completed
– $2.0 billion share repurchase completed, underscoring confidence in commercial portfolio and R&D pipeline
Conference Call and Webcast Scheduled Today at 8:00 a.m. ET
WILMINGTON, Del.--(BUSINESS WIRE)--Jul. 30, 2024-- Incyte (Nasdaq:INCY) today reports 2024 second quarter financial results, and provides a status update on the Company’s clinical development portfolio.
"In the second quarter of 2024, total revenues grew 9% year-over-year, surpassing $1.0 billion for the quarter. The commercial performance during this period was driven by strong patient demand for Opzelura® (ruxolitinib) and growth across all indications for Jakafi® (ruxolitinib)," said Hervé Hoppenot, Chief Executive Officer, Incyte. "In R&D, we completed a strategic review of our pipeline and have further intensified our focus on clinical programs that we believe can be transformational for patients. The $2.0 billion share repurchase completed during the second quarter, underscores our confidence in our commercial portfolio, clinical pipeline and Incyte's long-term value."
Transformation of Pipeline
-
Incyte announces a strategic review of its pipeline with an increased focus on high potential impact programs including, but not limited to:
IAI/Dermatology: povorcitinib and MRGPRX2 and MRGPRX4, which were recently acquired from Escient Pharmaceuticals
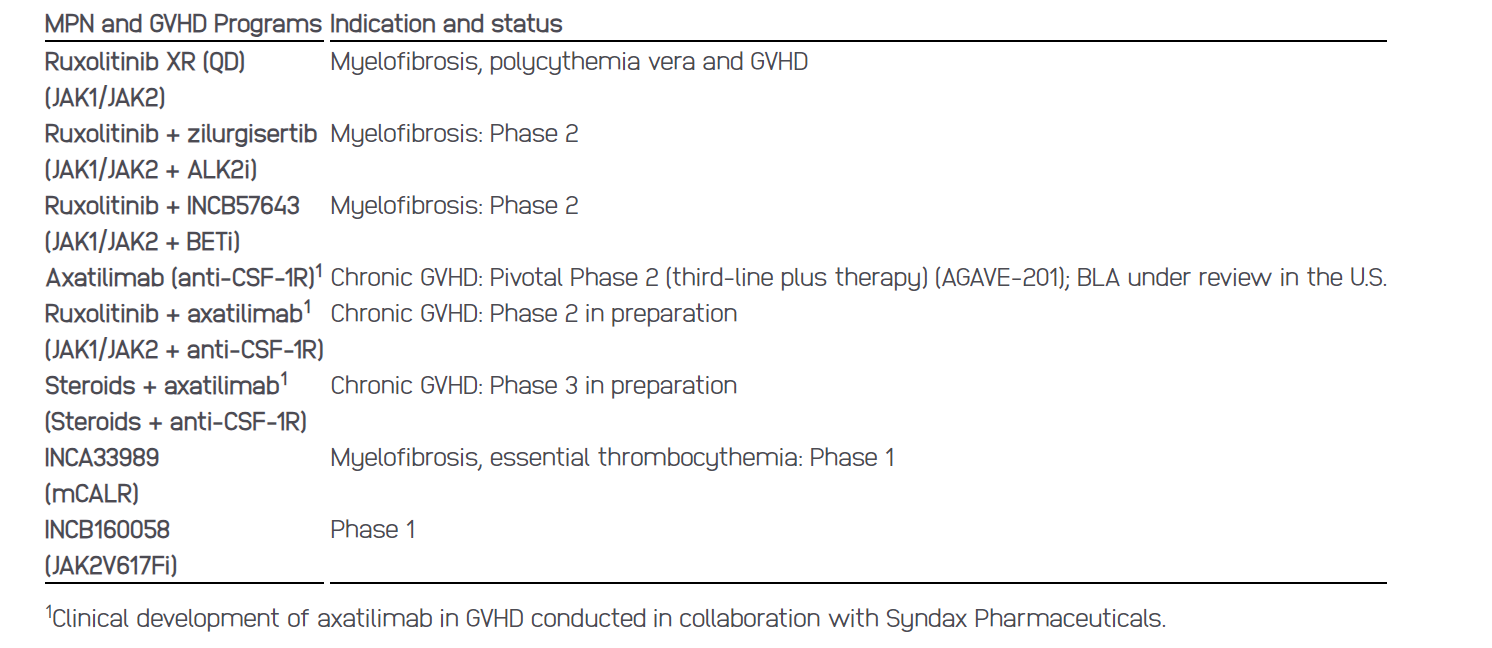
MPNs/GVHD: mCALR, JAK2V617Fi, BETi, and ALK2i
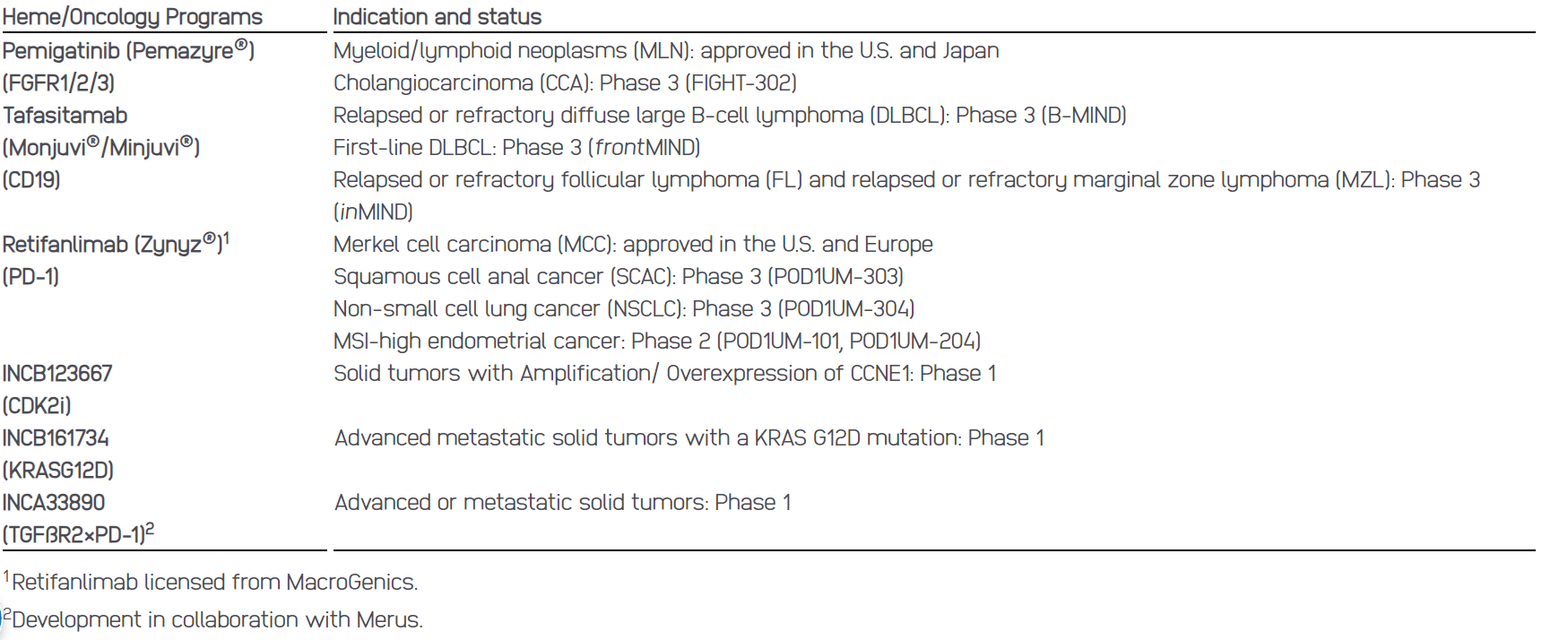
Oncology: CDK2i, KRASG12Di and TGFßR2×PD-1
-
The Company will discontinue further development of both oral, small molecule PD-L1 inhibitors, as well as LAG-3 monoclonal antibody, TIM-3 monoclonal antibody and LAG-3xPD-1 bispecific.
Recent Company Updates
-
Incyte announces positive topline results from two Phase 3 clinical studies evaluating retifanlimab (Zynyz®), a humanized monoclonal antibody targeting programmed cell death receptor-1 (PD-1), in squamous cell anal carcinoma (SCAC) and non-small cell lung cancer (NSCLC). The Phase 3 PODIUM-303 study in SCAC met its primary endpoint of progression free survival and the Phase 3 PODIUM-304 study in NSCLC met its primary endpoint of overall survival. The safety analysis from both studies demonstrated that retifanlimab was generally well-tolerated with no new safety signals observed.
-
Incyte plans to share the Phase 3 data from both studies in the second half of 2024. POD1UM-303 is a Phase 3, global, multicenter, randomized, double-blind study evaluating carboplatin-paclitaxel with retifanlimab or placebo in patients with inoperable locally recurrent or metastatic SCAC who have not previously been treated with chemotherapy. POD1UM-304 is a Phase 3, global, multicenter, randomized, double-blind study evaluating platinum-based chemotherapy with retifanlimab or placebo in patients with first-line, metastatic squamous or nonsquamous NSCLC.
-
In June 2024, Incyte repurchased a total of 33,325,849 shares of its common stock at a price of $60.00 per share, for a total cost of approximately $2.0 billion, excluding fees and expenses. These shares represented approximately 14.8 percent of the Company’s total outstanding shares of common stock as of June 7, 2024.
-
In May 2024, Incyte announced it completed the acquisition of Escient Pharmaceuticals, a clinical-stage drug discovery and development company advancing novel small molecule therapeutics for systemic immune and neuro-immune disorders. Under the terms of the agreement, Incyte acquired Escient and its clinical development portfolio, including EP262, a first-in-class, potent, highly selective, once-daily small molecule antagonist of Mas-related G protein-coupled receptor (MRGPRX2) and EP547, a first-in-class oral MRGPRX4 antagonist.
-
In April 2024, Incyte and China Medical System Holdings Limited announced the Companies entered into a Collaboration and License Agreement, through a wholly-owned dermatology medical aesthetic subsidiary CMS Skinhealth, for the development and commercialization of povorcitinib, a selective oral JAK1 inhibitor, in Mainland China, Hong Kong, Macau, Taiwan Region and eleven Southeast Asian countries.
Jakafi:
Net product revenues for the second quarter 2024 of $706 million (+3% Y/Y):
-
Paid demand increased 9% in the second quarter of 2024 versus the same quarter in the prior year, with growth across all indications.
-
Year over year net product revenue growth was lower than paid demand growth due to higher channel inventory levels at the end of the second quarter of 2023 versus the same period of 2024. Channel inventory at the end of the second quarter of 2024 was within the normal range.
Opzelura:
Net product revenues for the second quarter 2024 of $122 million (+52% Y/Y):
-
Net product revenues growth in the second quarter of 2024 were driven by patient demand, refills and expansion in payer coverage in both atopic dermatitis (AD) and vitiligo.
-
Net product revenues of $11 million in the second quarter of 2024 in Europe. Incyte achieved full reimbursement in Spain and Italy at the end of the second quarter 2024 and in France in July 2024.
Additional Pipeline Updates
Myeloproliferative Neoplasms (MPNs) and Graft-Versus-Host Disease (GVHD) – key highlights
-
Combination trials of ruxolitinib twice daily (BID) with zilurgisertib and BETi are ongoing and continue to enroll. A Phase 3 study for BETi is expected to advance into Phase 3 with an expected update later this year. Clinical proof-of-concept for zilurgisertib is anticipated in the second half of 2024.
-
The Phase 1 studies evaluating mCALR and JAK2V617Fi are ongoing and enrolling patients. Initial data for both studies is anticipated in 2025.
点击阅读原文了解更多详情