-
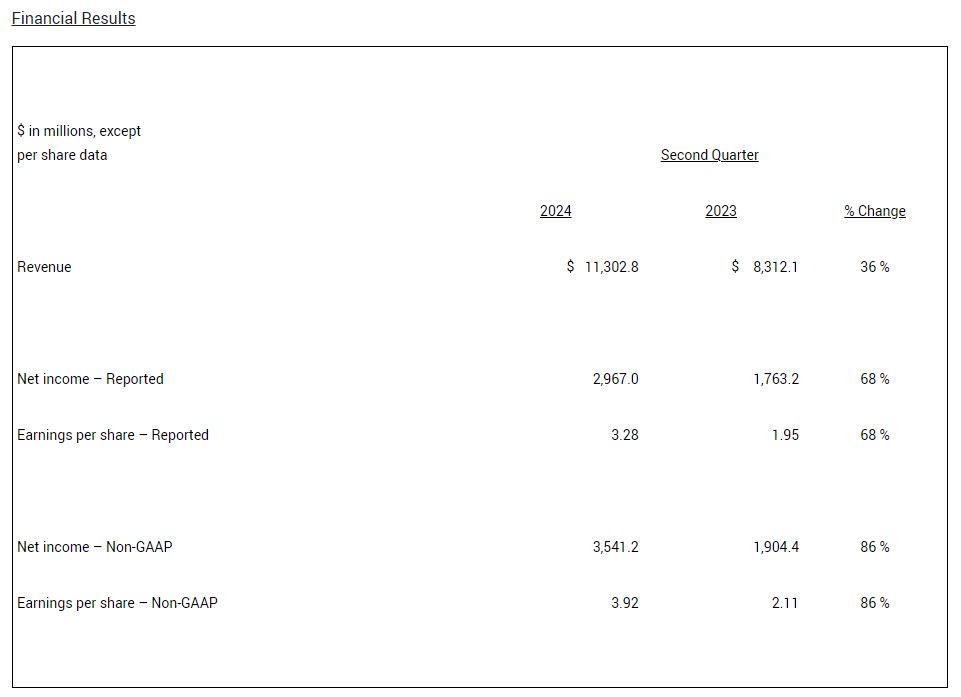
Revenue in Q2 2024 increased 36%, driven by Mounjaro, Zepbound and Verzenio. When excluding $579.0 million of revenue from the sale of rights for Baqsimi in Q2 2023, revenue in Q2 2024 increased 46%. Excluding the sale of rights for Baqsimi, non-incretin revenue increased 17% worldwide and 25% in the U.S.
-
Q2 2024 EPS increased 68% to $3.28 on a reported basis and increased 86% to $3.92 on a non-GAAP basis, both inclusive of $0.14 of acquired IPR&D charges.
-
2024 full-year revenue guidance raised by $3 billion; reported EPS guidance raised $2.05 to the range of $15.10 to $15.60, and non-GAAP EPS guidance raised $2.60 to the range of $16.10 to $16.60.
-
Pipeline progress included approval of Kisunla in the U.S. for Alzheimer's disease and Jaypirca in Japan for relapsed or refractory mantle cell lymphoma. Additional progress included submission of tirzepatide in the U.S. and EU for obstructive sleep apnea and obesity, and positive topline results from the Phase 3 trial evaluating tirzepatide for heart failure with preserved ejection fraction and obesity.
INDIANAPOLIS, Aug. 8, 2024 /PRNewswire/ -- Eli Lilly and Company (NYSE: LLY) today announced its financial results for the second quarter of 2024.
"Mounjaro, Zepbound and Verzenio led our strong financial performance in the second quarter as we advanced our manufacturing expansion agenda, and it is equally exciting to see the growth around the world of our medicines for cancer, neurological disorders and autoimmune diseases," said David A. Ricks, Lilly's chair and CEO. "We also recently received approval of Kisunla to help people with Alzheimer's disease, a moment that was decades in the making. Lilly's performance and progress in Alzheimer's, metabolic disorders and many other serious diseases highlight the tenacity, focus and capability of our scientists, clinicians, engineers, customer teams and collaborators."
Lilly shared numerous updates recently on key regulatory, clinical, business development and other events, including:
-
U.S. Food and Drug Administration (FDA) approval of Kisunla™ (donanemab-azbt) for the treatment of Alzheimer's disease;
-
Approval of Jaypirca® in Japan for people with relapsed or refractory mantle cell lymphoma who are resistant or intolerant to other Bruton tyrosine kinase inhibitors;
-
Submission of tirzepatide in the U.S. and EU for the treatment of moderate-to-severe obstructive sleep apnea in adults with obesity;
-
Submission of mirikizumab in Japanfor the treatment of moderately to severely active Crohn's disease;
-
Positive topline results from the SUMMIT Phase 3 clinical trial evaluating tirzepatide in adults with heart failure with preserved ejection fraction and obesity;
-
Positive topline results from the QWINT-2 and QWINT-4 Phase 3 clinical trials that showed once-a-week dosing of insulin efsitora alfa in adults with type 2 diabetes delivers A1C reduction and safety profile consistent with daily insulin;
-
The announcement of an agreement for Lilly to acquire Morphic Holding, Inc. to expand Lilly's immunology pipeline with oral integrin therapies for treatment of serious chronic diseases;
-
The commitment of an additional $5.3 billion manufacturing investment in the company's newest Indiana site to boost API production for tirzepatide and pipeline medicines;
-
The issuance of an open letter informing the public about potentially serious risks posed by the proliferation of counterfeit, fake, compounded, and other unsafe or untested versions of the company's FDA-approved tirzepatide medications and about the appropriate use of the company's authentic medicines; and
-
Announcements regarding changes to the company's executive leadership team.
For information on important public announcements, visit the news section of Lilly's website.
A discussion of the non-GAAP financial measures is included below under "Reconciliation of GAAP Reported to Selected Non-GAAP Adjusted Information (Unaudited)."
Second-Quarter Reported Results
In Q2 2024, worldwide revenue was $11.30 billion, an increase of 36% compared with Q2 2023, driven by a 27% increase in volume and a 10% increase due to higher realized prices, partially offset by a 1% decrease from the unfavorable impact of foreign exchange rates. The volume increase was primarily driven by growth from Mounjaro®, Zepbound®, Verzenio®, Taltz® and Jardiance®, partially offset by the sale of rights for Baqsimi® in Q2 2023 and declines in Trulicity®. Excluding $579.0 million of revenue from the sale of rights for Baqsimi in Q2 2023, revenue in Q2 2024 increased by 46%, and worldwide volume increased by 37%. Excluding the sale of rights for Baqsimi, non-incretin revenue increased 17% worldwide and 25% in the U.S.
Strong performance by the company's incretin medicines continued, as production increases resulted in improved channel dynamics and stocking levels in the U.S., contributing to sales growth during the quarter. While supply and demand have come into better balance, expected increases in demand may result in periodic supply tightness for certain presentations and dose levels. In the U.S., the company plans to launch Zepbound 2.5 mg and 5 mg single-dose vials in the coming weeks.
Higher realized prices were primarily driven by Mounjaro in the U.S., which saw net price positively impacted by access and savings card dynamics compared with Q2 2023. In the second half of 2024, these savings card dynamics should have a minimal impact on realized price comparisons to base periods, as the $25 non-covered benefit expired on June 30, 2023. New Products(i) revenue grew by $3.46 billion to $4.46 billion in Q2 2024, led by Mounjaro and Zepbound. Growth Products(ii) revenue increased 3% to $5.05 billion in Q2 2024 as growth led by Verzenio, Taltz, and Jardiance was largely offset by lower Trulicity sales.
(i) Lilly defines New Products as select products launched since 2022, which currently consist of Ebglyss, Jaypirca, Mounjaro, Omvoh and Zepbound.
(ii) Lilly defines Growth Products as select products launched prior to 2022, which currently consist of Cyramza, Emgality, Jardiance, Olumiant, Retevmo, Taltz, Trulicity, Tyvyt and Verzenio
Revenue in the U.S. increased 42% to $7.84 billion, driven by a 27% increase in volume and a 15% increase in realized prices. The increase in U.S. volume was driven by Zepbound, Mounjaro and Verzenio, partially offset by the sale of rights for Baqsimi in Q2 2023 and declines in Trulicity. The higher realized prices in the U.S. were primarily driven by Mounjaro. The company fulfilled the majority of prior incretin wholesaler backorders during Q2 2024, improving both wholesaler stocking levels and overall product availability for patients in the U.S. Q2 2024 Mounjaro and Zepbound sales in the U.S. were positively impacted by channel stocking that the company estimates totaled high teens to mid-20s as a percent of U.S. sales.
Revenue outside the U.S. increased 25% to $3.47 billion, driven by a 27% increase in volume, partially offset by a 3% decrease due to the unfavorable impact of foreign exchange rates. The increase in volume outside the U.S. was primarily driven by the launch of Mounjaro KwikPen® in various markets.
Gross margin increased 40% to $9.13 billion in Q2 2024. Gross margin as a percent of revenue was 80.8%, an increase of 2.5 percentage points. The increase in gross margin percent was primarily driven by favorable product mix and higher realized prices, partially offset by higher production costs.
In Q2 2024, research and development expenses increased 15% to $2.71 billion, or 24% of revenue, driven by continued investments in the company's portfolio and its people.
Marketing, selling and administrative expenses increased 10% to $2.12 billion in Q2 2024, primarily driven by investments in the company's launches and its people.
In Q2 2024, the company recognized acquired in-process research and development (IPR&D) charges of $154.3 million compared with $97.1 million in Q2 2023.
Asset impairment, restructuring and other special charges were $435.0 million in Q2 2024, which was related to anticipated litigation payments. There were no asset impairment, restructuring and other special charges in Q2 2023.
Other income (expense) was expense of $197.6 million in Q2 2024, compared to expense of $36.8 million in Q2 2023. The increase in expense was primarily driven by larger net losses on investments in equity securities in Q2 2024 and higher net interest expenses.
The effective tax rate was 15.6% in both Q2 2024 and Q2 2023. The Q2 2024 tax rate reflects a mix of earnings in higher tax jurisdictions, while the Q2 2023 rate reflects the impact of earnings from the sale of rights for Baqsimi.
In Q2 2024, net income and earnings per share (EPS) were $2.97 billion and $3.28, respectively, compared with net income of $1.76 billion and EPS of $1.95 in Q2 2023. EPS in Q2 2024 included $0.14 of acquired IPR&D charges compared with $0.09 in Q2 2023.
Mounjaro
For Q2 2024, worldwide Mounjaro revenue was $3.09 billion compared with $979.7 million in Q2 2023. U.S. revenue was $2.41 billion compared with $915.7 million in Q2 2023, reflecting continued strong demand, improved channel dynamics, and higher realized prices due to savings card dynamics. In the second half of 2024, these savings card dynamics should have a minimal impact on realized price comparisons to base periods, as the $25 non-covered benefit expired on June 30, 2023. Revenue outside the U.S. increased to $677.2 million compared with $64.0 million in Q2 2023, primarily driven by volume associated with the launch of Mounjaro KwikPen in various markets.
Trulicity
For Q2 2024, worldwide Trulicity revenue decreased 31% compared with Q2 2023 to $1.25 billion. U.S. revenue decreased 36% to $876.7 million, driven by decreased sales volume primarily due to competitive dynamics and supply constraints, partially offset by improved wholesaler stocking levels on certain doses. Revenue outside the U.S. decreased 16% to $368.9 million, primarily driven by decreased volume. In addition to the factors affecting U.S. volume, international markets continue to be impacted by actions Lilly has taken to manage demand amid tight supply, including measures to minimize the impact on existing patients by communicating with healthcare practitioners to not start new patients on Trulicity.
Verzenio
For Q2 2024, worldwide Verzenio revenue increased 44% compared with Q2 2023 to $1.33 billion. U.S. revenue was $861.4 million, an increase of 46%, primarily driven by increased demand. Revenue outside the U.S. was $470.5 million, an increase of 39%, driven by increased demand, partially offset by the unfavorable impact of foreign exchange rates.
Zepbound
For Q2 2024, U.S. Zepbound revenue was $1.24 billion. Zepbound launched in the U.S. for the treatment of adult patients with obesity or overweight with weight-related comorbidities in November 2023.
Jardiance
For Q2 2024, the company's worldwide Jardiance revenue increased 15% compared with Q2 2023 to $769.6 million. U.S. revenue was $428.9 million, an increase of 11%, driven by increased demand. Revenue outside the U.S. was $340.7 million, an increase of 21%, driven by increased volume.
Jardiance is part of the company's alliance with Boehringer Ingelheim. Lilly reports as revenue royalties received on net sales of Jardiance.
Taltz
For Q2 2024, worldwide Taltz revenue increased 17% compared with Q2 2023 to $824.7 million. U.S. revenue increased 14% to $539.4 million, driven by increased demand and, to a lesser extent, channel dynamics. Revenue outside the U.S. increased 23% to $285.3 million, driven by increased demand.
Humalog
For Q2 2024, worldwide Humalog revenue increased 43% compared with Q2 2023 to $631.6 million. U.S. revenue was $434.7 million, an increase of 89%, driven by higher realized prices primarily due to changes to estimates for rebates and discounts, segment mix and increased demand. Revenue outside the U.S. was $196.9 million, a decrease of 7%, driven by decreased volume, partially offset by higher realized prices.
About Lilly
Lilly is a medicine company turning science into healing to make life better for people around the world. We've been pioneering life-changing discoveries for nearly 150 years, and today our medicines help more than 51 million people across the globe. Harnessing the power of biotechnology, chemistry and genetic medicine, our scientists are urgently advancing new discoveries to solve some of the world's most significant health challenges: redefining diabetes care; treating obesity and curtailing its most devastating long-term effects; advancing the fight against Alzheimer's disease; providing solutions to some of the most debilitating immune system disorders; and transforming the most difficult-to-treat cancers into manageable diseases. With each step toward a healthier world, we're motivated by one thing: making life better for millions more people. That includes delivering innovative clinical trials that reflect the diversity of our world and working to ensure our medicines are accessible and affordable. To learn more, visit Lilly.com and Lilly.com/news. F-LLY