-
87.5% of patients treated with LYNPARZA were alive at six-years vs. 83.2% in the comparator arm
-
First and only PARP inhibitor to improve overall survival in early breast cancer
December 11, 2024 10:22 am ET--RAHWAY, N.J.--(BUSINESS WIRE)-- AstraZeneca and Merck (NYSE: MRK), known as MSD outside of the United States and Canada, today announced long-term results from the OlympiA Phase 3 trial which showed LYNPARZA (olaparib) demonstrated sustained, clinically meaningful improvements in overall survival (OS), invasive disease-free survival (IDFS) and distant disease-free survival (DDFS) for people with germline BRCA-mutated (gBRCAm) HER2-negative high-risk early breast cancer.
These results were presented today at the 2024 San Antonio Breast Cancer Symposium (#GS1-09) and were consistent with positive primary results published in The New England Journal of Medicine .
Judy E. Garber, Chief of the Division of Cancer Genetics and Prevention at Dana-Farber Cancer Institute and co-principal investigator of the trial said, “These exciting long-term data from OlympiA confirm that adjuvant treatment with olaparib for one year continues to deliver clinically meaningful survival benefit for patients with germline BRCA-mutated high-risk HER2-negative early breast cancer even after six years, with benefit persisting in all subgroups and with toxicity and pregnancy data reassuring for this generally younger group. These data reinforce the importance of germline BRCA testing at the time of diagnosis, so we can identify all eligible patients who may benefit from treatment with olaparib as early as possible.”
Breast cancer is the second most diagnosed cancer worldwide, with an estimated 2.3 million patients diagnosed in 2022. About 63% of all breast cancer patients are diagnosed at an early stage of disease and BRCA mutations are found in approximately 5-10% of patients.
Susan Galbraith, Executive Vice President, Oncology R&D, AstraZeneca, said: “Two years ago, LYNPARZA became the first and only PARP inhibitor to demonstrate a survival benefit in germline BRCA - mutated, HER2-negative and high-risk early-stage breast cancer. To see this benefit continue after six years of follow-up is tremendous for patients and reinforces how LYNPARZA is continuing to transform the treatment of BRCA - mutated early-stage breast cancer.”
Dr. Eliav Barr, senior vice president, head of global clinical development and chief medical officer, Merck Research Laboratories, said: “The durable long-term efficacy seen in the OlympiA study reinforces LYNPARZA as an important treatment option for those living with this truly challenging, very aggressive form of breast cancer.”
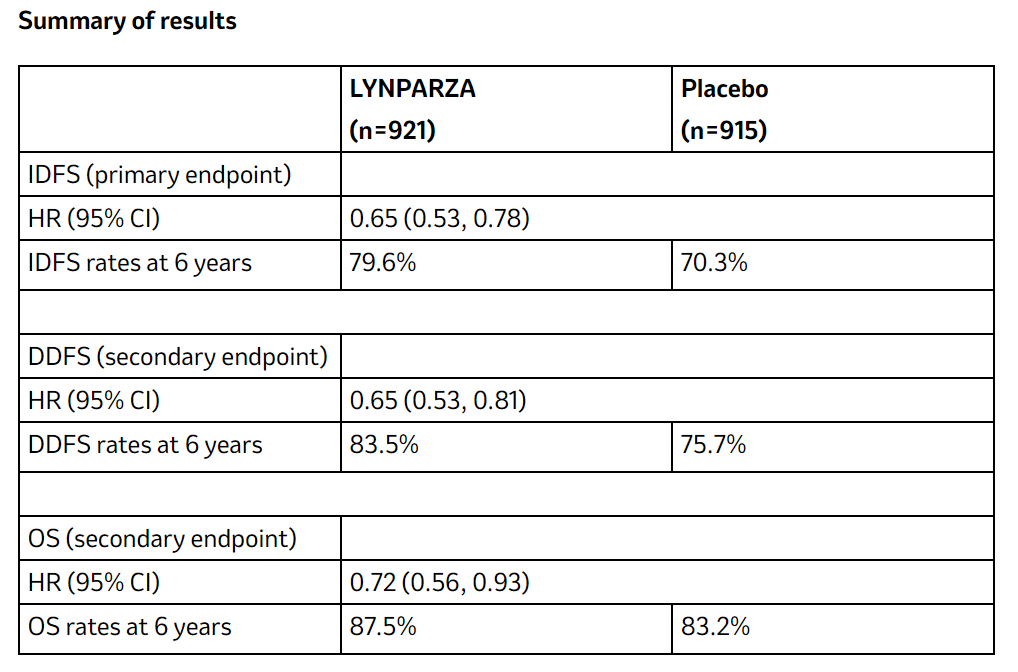
At a median follow-up of 6.1 years (maximum 9.6 years) in eligible patients, who had completed local treatment and standard neoadjuvant or adjuvant chemotherapy, LYNPARZA reduced the risk of death by 28% (hazard ratio [HR] 0.72; 95% confidence interval [CI] 0.56-0.93) versus placebo. In addition, 87.5% of patients treated with LYNPARZA remained alive versus 83.2% of those on placebo.
LYNPARZA also demonstrated sustained and clinically meaningful improvements in the primary and secondary endpoints of IDFS and DDFS. LYNPARZA reduced the risk of invasive breast cancer recurrence, second cancers or death by 35% (HR 0.65; 95% CI; 0.53-0.78) and reduced the risk of distant disease recurrence or death by 35% (HR 0.65; 95% CI; 0.53-0.81) versus placebo. The benefit with LYNPARZA was consistent across all key subgroups, including patients with high-risk, hormone-receptor-positive disease.
The safety and tolerability profile of LYNPARZA in this trial was in line with that observed in prior clinical trials and no new safety signals were identified with longer follow-up. No evidence of an increased risk of myelodysplastic syndrome or acute myeloid leukemia was observed compared to those on placebo.
The OlympiA trial is coordinated by the Breast International Group (BIG) in partnership with NRG Oncology, the US National Cancer Institute (NCI), the Frontier Science & Technology Research Foundation (FSTRF), AstraZeneca and Merck. 5
LYNPARZA is approved in the United States (US), European Union (EU), Japan, and many other countries for the treatment of gBRCAm, HER2-negative high-risk early breast cancer based on the results of the OlympiA Phase 3 trial. LYNPARZA is also approved in the US, EU, Japan, and many other countries for the treatment of patients with gBRCAm, HER2-negative, metastatic breast cancer. In the EU, this indication also includes patients with locally advanced breast cancer.
About OlympiA
OlympiA is a phase III, double-blind, parallel group, placebo-controlled, multicenter trial evaluating the efficacy and safety of LYNPARZA tablets versus placebo as a 12-month adjuvant treatment for adult patients with gBRCAm HER2-negative early breast cancer, who have completed neoadjuvant or adjuvant chemotherapy. 5 The primary endpoint of the trial is invasive disease-free survival defined as time from randomization to date of first loco-regional or distant recurrence or new cancer or death from any cause. Key secondary endpoints include distant disease-free survival and overall survival, which is defined as time from randomization until documented evidence of first distant recurrence of breast cancer or death without distant recurrence. 5
About LYNPARZA ® (olaparib)
LYNPARZA is a first-in-class PARP inhibitor and the first targeted treatment to potentially exploit DNA damage response (DDR) pathway deficiencies, such as BRCA mutations, to preferentially kill cancer cells. Inhibition of PARP with LYNPARZA leads to the trapping of PARP bound to DNA single-strand breaks, stalling of replication forks, their collapse and the generation of DNA double-strand breaks and cancer cell death. LYNPARZA is being tested in a range of tumor types with defects and dependencies in the DDR.
LYNPARZA, which is being jointly developed and commercialized by AstraZeneca and Merck, has a broad and advanced clinical trial development program, and AstraZeneca and Merck are working together to understand how it may affect multiple PARP-dependent tumors as a monotherapy and in combination across multiple cancer types.
About BRCA Mutations
BRCA 1 and BRCA 2 (breast cancer susceptibility genes 1/2) are human genes that produce proteins responsible for repairing damaged DNA and play an important role maintaining the genetic stability of cells. When either of these genes is mutated or altered such that its protein product either is not made or does not function correctly, DNA damage may not be repaired properly, and cells become unstable. As a result, cells are more likely to develop genetic alterations that can lead to cancer.
About breast cancer
Early breast cancer is defined as disease confined to the breast with or without regional lymph node involvement, and the absence of distant metastatic disease. In the US, the 5-year survival rate is 99.6% for localized breast cancer (only found in the breast area) and 86.7% for regional breast cancer (cancer that has spread outside the breast to nearby structures or lymph nodes). Despite advancements in the treatment of early breast cancer, up to 30% of patients with high-risk clinical and/or pathologic features recur within the first few years and patients with g BRCA mutations are more likely to be diagnosed at a younger age than those without these mutations. Breast cancer is one of the most biologically diverse tumor types with various factors fuelling its development and progression. The discovery of biomarkers in the development of breast cancer has greatly impacted scientific understanding of the disease.
About the AstraZeneca and Merck strategic oncology collaboration
In July 2017, AstraZeneca and Merck, known as MSD outside the US and Canada, announced a global strategic oncology collaboration to co-develop and co-commercialize certain oncology products including LYNPARZA, the world’s first PARP inhibitor, for multiple cancer types. Working together, the companies will develop these products in combination with other potential new medicines and as monotherapies. Independently, the companies will develop these oncology products in combination with their respective PD-L1 and PD-1 medicines.
Merck’s focus on cancer
Our goal is to translate breakthrough science into innovative oncology medicines to help people with cancer worldwide. At Merck, the potential to bring new hope to people with cancer drives our purpose and supporting accessibility to our cancer medicines is our commitment. As part of our focus on cancer, Merck is committed to exploring the potential of immuno-oncology with one of the largest development programs in the industry across more than 30 tumor types. We also continue to strengthen our portfolio through strategic acquisitions and are prioritizing the development of several promising oncology candidates with the potential to improve the treatment of advanced cancers. For more information about our oncology clinical trials, visit www.merck.com/clinicaltrials.
About Merck
At Merck, known as MSD outside of the United States and Canada, we are unified around our purpose: We use the power of leading-edge science to save and improve lives around the world. For more than 130 years, we have brought hope to humanity through the development of important medicines and vaccines. We aspire to be the premier research-intensive biopharmaceutical company in the world – and today, we are at the forefront of research to deliver innovative health solutions that advance the prevention and treatment of diseases in people and animals. We foster a diverse and inclusive global workforce and operate responsibly every day to enable a safe, sustainable and healthy future for all people and communities.