- As of February 17, 2025, 1,028 unique patient prescriptions for Attruby™ have been written by 516 unique prescribers since FDA approval
- Attruby (acoramidis), the first and only near-complete TTR stabilizer (≥90%) was approved by the FDA to reduce cardiovascular death and cardiovascular-related hospitalization in ATTR-CM patients on November 22, 2024
- Acoramidis was approved as BEYONTTRA™ in the EU on February 10, 2025, achieving a $75 million milestone payment and ongoing royalties in a tiered structure beginning in the low-thirties percent on sales in the EU
- Acoramidis demonstrated a 59% hazard reduction on the composite endpoint of all-cause mortality and first cardiovascular-related hospitalization in the variant ATTR-CM population by month 30; to the Company's knowledge, this benefit is the largest and the only statistically significant result in this patient population, which has an aggressive phenotype and poor prognosis
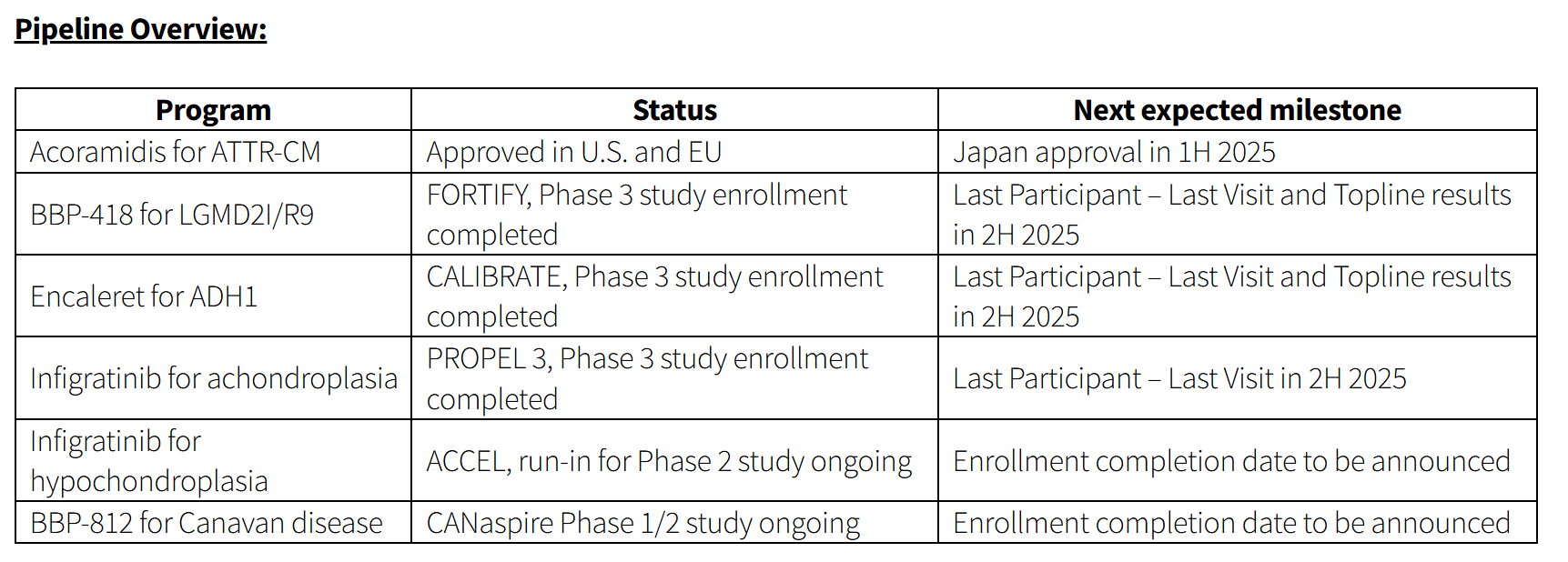
- Fully enrolled three global registrational studies – FORTIFY (BBP-418 for LGMD2I/R9), CALIBRATE (encaleret for ADH1), and PROPEL 3 (infigratinib for achondroplasia) – with last participant – last visit expected for each study before the end of 2025
- The Company ended the fourth quarter with $681 million in cash, cash equivalents, and short-term restricted cash. Further, the Company expects to receive $105 million in regulatory milestones in 1H 2025 from acoramidis Europe and Japan approvals
PALO ALTO, Calif., Feb. 20, 2025 (GLOBE NEWSWIRE) -- BridgeBio Pharma, Inc. (Nasdaq: BBIO) (“BridgeBio” or the “Company”), a new type of biopharmaceutical company focused on genetic diseases announced today its financial results for the fourth quarter and full year ended December 31, 2024, and provided an update on Attruby’s commercial progress.
Commercial Progress:
As of February 17, 2025, 1,028 unique patient prescriptions for Attruby have been written by 516 unique healthcare providers since FDA approval.
”I am very encouraged by the strength of the Attruby launch, with prescriptions being successfully filled across all patient types,” said Matt Outten, Chief Commercial Officer of BridgeBio. “In conversations with healthcare providers and patients, we have repeatedly heard that Attruby's category-leading results - time to separation of just three months, along with a 42% reduction in all-cause mortality and recurrent hospitalizations and a 50% reduction in cardiovascular hospitalizations at 30 months - set it apart as a clinically meaningful advancement for ATTR-CM. Combined with our industry-leading patient support programs, we believe Attruby is delivering a much-needed change in the treatment landscape.”
Key Program Updates:
“It is exciting to see patients, physicians, and payers resonate with our message that the greater levels of TTR stabilization that Attruby delivers can be of benefit to the patients we serve and that the TTR protein is clinically important, not toxic.” said Neil Kumar, Ph.D., Founder and CEO of BridgeBio. “We look forward to continuing to partner with the community to ensure that we find all patients that can be helped and ease their path to getting on therapy, when appropriate, as much as possible.”
Attruby (acoramidis) – the first approved, near-complete (≥90%) TTR stabilizer for treatment of transthyretin amyloid cardiomyopathy (ATTR-CM):
On November 22, 2024, the U.S. Food and Drug Administration (FDA) approved Attruby (acoramidis), a near-complete TTR stabilizer (≥90%), to reduce cardiovascular death and cardiovascular-related hospitalization (CVH) in adult patients with ATTR-CM.
On February 10, 2025, the European Commission approved BEYONTTRA (acoramidis) for use in adult patients with ATTR-CM in the EU.
Preliminary results from the ongoing ATTRibute-CM open-label extension (OLE) study of Attruby in ATTR-CM were simultaneously published in Circulation and presented at the American Heart Association Scientific Sessions, showing that Attruby demonstrated statistically significant risk reduction of 36% on All-Cause Mortality (ACM) alone at month 36 within the OLE, and 46% (p<0.0001) and 48% (p<0.0001) reductions in the composite endpoint of ACM and recurrent CVH at months 36 and 42, respectively.
Attruby is supported by industry-leading access programs designed to ensure seamless treatment initiation and continuity for all patients with ATTR-CM.
BBP-418 – Glycosylation substrate in development for limb-girdle muscular dystrophy type 2I/R9 (LGMD2I/R9):
FORTIFY, the Phase 3 clinical trial of BBP-418 in LGMD2I/R9, a rare genetic disorder caused by variants in the fukutin‑related protein (FKRP) gene, is fully enrolled with 112 participants. The trial is the largest prospective interventional study to ever be conducted in LGMD2I.
The Company expects to achieve last participant – last visit and report topline results of the interim analysis cohort in the second half of 2025.
If successful, we expect BBP-418 would be the first approved therapy for individuals living with LGMD2I/R9.
Encaleret – Calcium-sensing receptor (CaSR) antagonist in development for autosomal dominant hypocalcemia type 1 (ADH1) and postsurgical hypoparathyroidism (PSH):
CALIBRATE, the Phase 3 clinical trial of encaleret in ADH1, a genetic form of hypoparathyroidism, is fully enrolled with 71 participants. The trial is the largest prospective interventional study to ever be conducted in ADH1.
The Company expects to achieve last participant – last visit and report topline results in the second half of 2025.
If successful, we expect encaleret would be the first approved therapy indicated for individuals living with ADH1.
A Phase 2 study of encaleret in PSH is ongoing, with preliminary evidence suggestive of a differentiated profile for encaleret in PSH.
Infigratinib – FGFR1-3 inhibitor in development for achondroplasia and hypochondroplasia:
PROPEL 3, the Phase 3 clinical trial of infigratinib in achondroplasia, the most common form of disproportionate short stature, is fully enrolled with 114 participants randomized.
The Company expects to achieve last participant – last visit in the second half of 2025.
In November 2024, the Phase 2 PROPEL 2 study of infigratinib in children with achondroplasia was published in the New England Journal of Medicine.
If successful, we expect infigratinib would be the first approved oral therapy option for children living with achondroplasia.
The Company is currently enrolling the ACCEL run-in for a Phase 2 study of infigratinib in hypochondroplasia.
Financial Updates:
Cash, Cash Equivalents, and Short-term Restricted Cash
Cash, cash equivalents and short-term restricted cash, totaled $681.2 million as of December 31, 2024, compared to $392.6 million of cash, cash equivalents and short-term restricted cash as of December 31, 2023. The $288.6 million net increase in cash, cash equivalents and short-term restricted cash was primarily attributable to net proceeds received from the Funding Agreement of $488.8 million, net proceeds received from the term loan under the credit facility of $434.0 million, net proceeds received from various equity financings of $314.7 million, proceeds from the sale of investments in equity securities of $63.2 million, and special cash dividends received from investments in equity securities of $25.7 million. These increases in cash, cash equivalents and short-term restricted cash were primarily offset by the impacts of net cash used in operating activities of $520.7 million, refinancing the Company’s previous senior secured credit term loan, inclusive of prepayment fees and exit-related costs in aggregate of $473.4 million, purchases of equity securities of $20.3 million, Funding Agreement transaction related costs of $16.3 million, and the repurchase of shares to satisfy tax withholdings of $7.5 million during the year ended December 31, 2024.
Revenue
Revenue for the three months and year ended December 31, 2024, was $5.9 million and $221.9 million, respectively, as compared to $1.7 million and $9.3 million for the same periods in the prior year.
The increase of $4.2 million in revenue for the three months ended December 31, 2024, compared to the same period in the prior year, was primarily due to the recognition of $2.9 million in net product revenue from the first commercial sales of Attruby in the U.S. following the FDA approval on November 22, 2024, and services revenue received under the exclusive license and collaboration agreements with Bayer and Kyowa Kirin. Revenue for the three months ended December 31, 2023, primarily consisted of the recognition of services revenue under the Navire-BMS License Agreement, which terminated in June 2024.
The increase of $212.6 million in revenue for the year ended December 31, 2024, compared to the same period in the prior year, was primarily due to $207.7 million from recognition of the upfront payments and service revenue under the Bayer and the Kyowa Kirin exclusive license and collaboration agreements, and $2.9 million in net product revenue from the first commercial sales of Attruby following the FDA approval on November 22, 2024.
Operating Costs and Expenses
Operating costs and expenses for the three months and year ended December 31, 2024, were $231.9 million and $814.9 million, respectively, compared to $179.2 million and $616.7 million for the same periods in the prior year.
The overall increase of $52.7 million, in operating costs and expenses for the three months ended December 31, 2024, compared to the same period in the prior year, was primarily due to an increase of $47.2 million in selling, general and administrative (SG&A) expenses mainly to support commercialization of Attruby, which included costs incurred for marketing, advertising and hiring of a sales force in the U.S., an increase of $3.9 million in restructuring, impairment and related charges, and an increase of $1.6 million in research and development (R&D) expenses to advance the Company’s pipeline of R&D programs.
The overall increase of $198.2 million, in operating costs and expenses for the year ended December 31, 2024, compared to the same period in the prior year, was primarily due to an increase of $138.3 million in SG&A expenses related to costs primarily to support the commercial launch of Attruby which included costs incurred for marketing, advertising and hiring of a sales force in the U.S., an increase of $52.2 million in R&D expenses to advance the Company’s pipeline of R&D programs, and an increase of $7.7 million in restructuring, impairment and related charges. Operating costs and expenses for the year ended December 31, 2024, include $25.0 million of nonrecurring deal-related costs for transactions that were completed during the year ended December 31, 2024.
Restructuring, impairment and related charges for the three months and year ended December 31, 2024, amounted to $4.7 million and $15.6 million, respectively. These charges primarily consisted of impairments and write-offs of long-lived assets, severance and employee-related costs, and exit and other related costs. Restructuring, impairment, and related charges for the same periods in the prior year were $0.8 million and $7.9 million, respectively. These charges primarily consisted of winding down, exit costs, and severance and employee-related costs.
Stock-based compensation expenses included in operating costs and expenses for the three months ended December 31, 2024, were $36.4 million, of which $20.0 million is included in R&D expenses, $16.3 million is included in SG&A expenses, and less than $0.1 million is included in restructuring, impairment, and related charges. Stock-based compensation expenses included in operating costs and expenses for the same period in the prior year were $37.1 million, of which $22.5 million is included in R&D expenses, and $14.6 million is included in SG&A expenses.
Stock-based compensation expenses included in operating costs and expenses for the year ended December 31, 2024, were $113.9 million, of which $63.9 million is included in SG&A expenses, $49.8 million is included in R&D expenses, and $0.2 million is included in restructuring, impairment and related charges. Stock-based compensation expenses included in operating costs and expenses for the same period in the prior year were $115.0 million, of which $61.6 million is included in R&D expenses, and $53.4 million is included in SG&A expenses.
Total Other Income (Expense), net
Total other income (expense), net for the three months and year ended December 31, 2024, were ($40.2) million and $50.8 million, respectively, compared to $7.1 million and ($45.9) million for the same periods in the prior year.
The increase in total other expense, net of $47.3 million for the three months ended December 31, 2024, compared to the same period in the prior year, was primarily due to a decrease in other income, net of $20.1 million mainly due to market fair value adjustments from the Company’s investments in equity securities, a net loss from equity method investments of $16.7 million, an increase in interest expense, net of $9.6 million, and a decrease in interest income of $0.9 million.
The increase in total other income, net of $96.7 million for the year ended December 31, 2024 , compared to the same period in the prior year, was primarily due to gains the Company recognized on the deconsolidation of subsidiaries of $178.3 million. These gains were partially offset by recognition a net loss from equity method investments of $31.2 million, a loss on extinguishment of debt of $26.6 million, an increase in interest expense, net of $18.0 million, a decrease in other income, net of $5.0 million mainly due to market fair value adjustments from the Company’s investments in equity securities, and a decrease in interest income of $0.8 million.
Net Loss Attributable to Common Stockholders of BridgeBio and Net Loss per Share
For the three months and year ended December 31, 2024, the Company recorded a net loss attributable to common stockholders of BridgeBio of $265.1 million and $535.8 million, respectively, compared to $168.1 million and $643.2 million, respectively, for the three months and year ended December 31, 2023.
For the three months and year ended December 31, 2024, the Company reported a net loss per share of $1.40 and $2.88, respectively, compared to $0.96 and $3.95, respectively, for the three months and year ended December 31, 2023.
About BEYONTTRA™ (acoramidis)
On 10 February 2025, the European Commission granted Marketing Authorization for BEYONTTRA™ (acoramidis) for the treatment of wild-type or variant transthyretin amyloidosis in adult patients with cardiomyopathy (ATTR-CM). For full prescribing information, please refer to the Summary of Product Characteristics (SmPC).
About BridgeBio Pharma, Inc.
BridgeBio Pharma (BridgeBio) is a new type of biopharmaceutical company founded to discover, create, test, and deliver transformative medicines to treat patients who suffer from genetic diseases. BridgeBio’s pipeline of development programs ranges from early science to advanced clinical trials. BridgeBio was founded in 2015 and its team of experienced drug discoverers, developers and innovators are committed to applying advances in genetic medicine to help patients as quickly as possible.