May 15, 2025--FOSTER CITY, Calif. & SANTA MONICA, Calif.--(BUSINESS WIRE)-- Gilead Sciences, Inc. (Nasdaq: GILD) will present more than 20 abstracts across both Gilead and Kite at the upcoming 2025 American Society of Clinical Oncology (ASCO) Annual Meeting May 30 – June 3 and the 2025 European Hematology Association (EHA) Annual Congress June 12 – 15. The studies span breast cancer and other solid tumors (glioblastoma, endometrial cancer, lung cancer, gastric cancer), as well as multiple blood cancers (multiple myeloma, large B-cell lymphoma, indolent non-Hodgkin lymphoma, acute lymphoblastic leukemia).
At ASCO, Gilead will present detailed late-breaking results from the Phase 3 ASCENT-04 study showing a statistically significant and clinically meaningful benefit in progression-free survival for Trodelvy® plus Keytruda® versus Keytruda and standard of care chemotherapy in patients with inoperable (unresectable) locally advanced or metastatic triple-negative breast cancer whose tumors express PD-L1 (Abstract #LBA109). Additionally, Kite research collaborators at the University of Pennsylvania Perelman School of Medicine will present Phase 1 results evaluating a novel investigational CAR T-cell therapy using a dual-target approach in patients with recurrent glioblastoma during an oral session at ASCO (Abstract #102).
At EHA, Kite and its partner Arcellx will present updated findings from the Phase 2 registrational iMMagine-1 study of anitocabtagene-autoleucel (anito-cel) in relapsed/refractory multiple myeloma during an oral presentation (Abstract #S201).
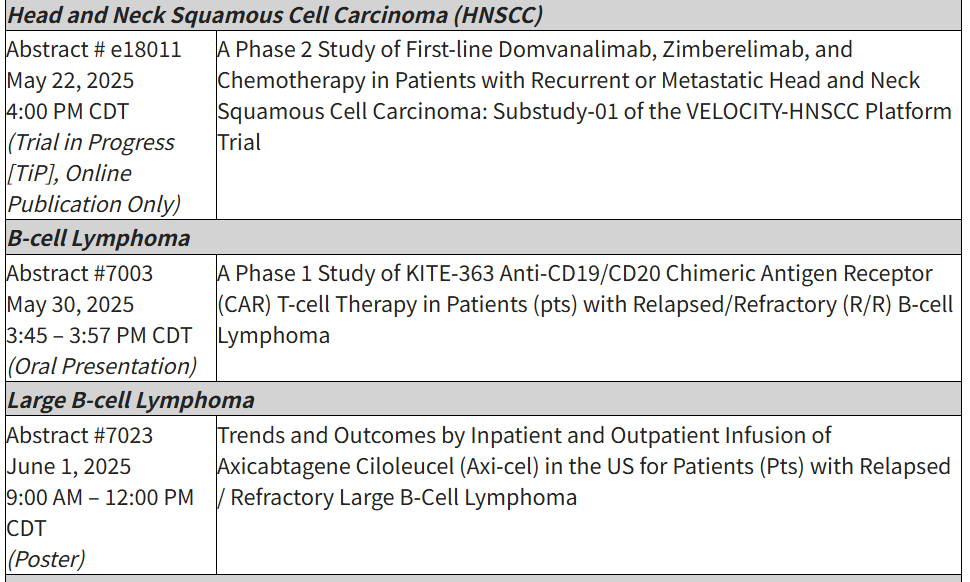
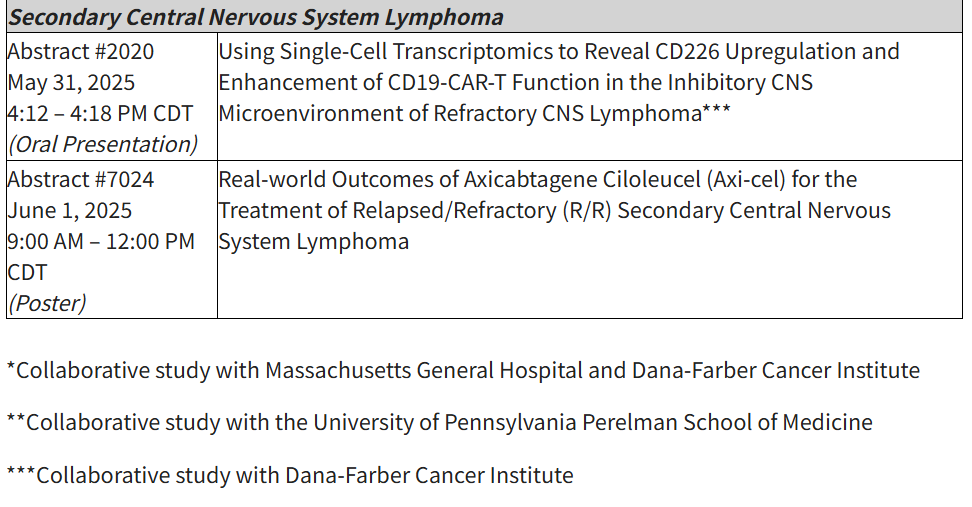
Additional abstracts supporting pipeline therapies (e.g., KITE-363, a C19/20 dual-target CAR T) and results from collaborative studies will also be presented as orals across ASCO and EHA.
“Our oncology portfolio is broad and diverse by design, as we continue to innovate with next-generation therapies and combinations to deliver improved outcomes and ultimately seek to transform how cancer is treated,” said Dietmar Berger, MD, PhD, Chief Medical Officer, Gilead Sciences. “Data at ASCO and EHA will feature novel pipeline approaches with antibody-drug conjugate therapy and cell therapy, helping to drive oncology innovation and change medical practice.”
Summary of Presentations
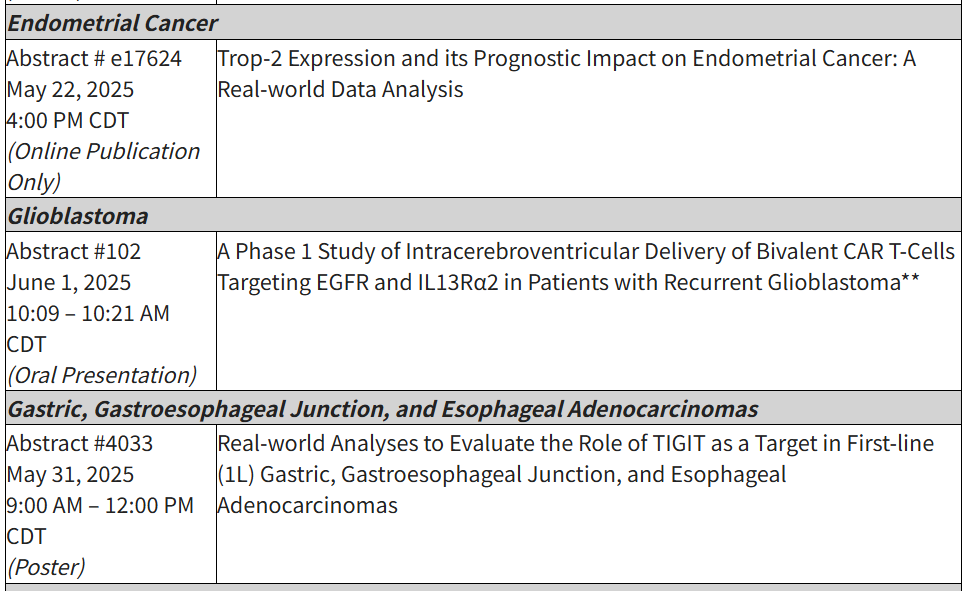
Accepted abstracts at the 2025 ASCO Annual Meeting include: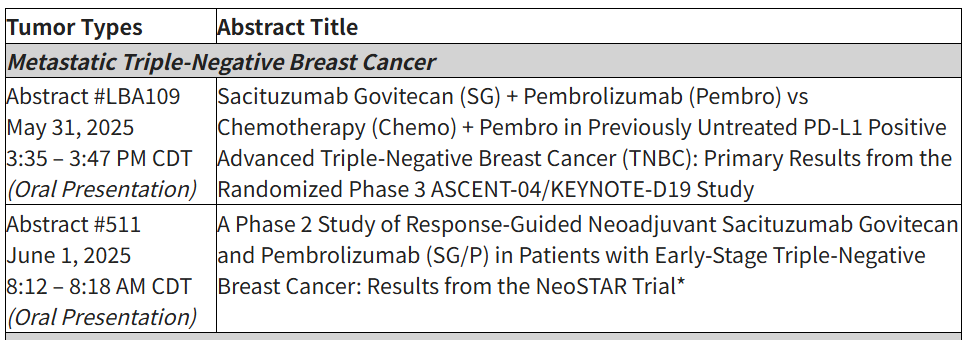

About Trodelvy
Trodelvy® (sacituzumab govitecan-hziy) is a first-in-class Trop-2-directed antibody-drug conjugate. Trop-2 is a cell surface antigen highly expressed in multiple tumor types, including in more than 90% of breast and lung cancers. Trodelvy is intentionally designed with a proprietary hydrolyzable linker attached to SN-38, a topoisomerase I inhibitor payload. This unique combination delivers potent activity to both Trop-2 expressing cells and the tumor microenvironment through a bystander effect.
Trodelvy is currently approved in more than 50 countries for second-line or later metastatic triple-negative breast cancer (TNBC) patients and in more than 40 countries for certain patients with pre-treated HR+/HER2- metastatic breast cancer.
Trodelvy is currently being evaluated in multiple ongoing Phase 3 trials across a range of tumor types with high Trop-2 expression. These studies with Trodelvy, both in monotherapy and in combination with pembrolizumab, involve earlier lines of treatment for TNBC and HR+/HER2- breast cancer—including in curative settings—as well as in lung and gynecologic cancers, where previous proof-of-concept studies have demonstrated clinical activity.
About Anitocabtagene autoleucel (anito-cel)
Anitocabtagene autoleucel (anito-cel, previously CART-ddBCMA) is the first BCMA-directed CAR T-cell therapy to be investigated in multiple myeloma that utilizes Arcellx’s novel and compact binder known as the D-Domain. Anito-cel has been granted Fast Track, Orphan Drug, and Regenerative Medicine Advanced Therapy Designations by the U.S. Food and Drug Administration.
About Arcellx and Kite Collaboration
Arcellx and Kite, a Gilead Company, formed a global strategic collaboration to co-develop and
co-commercialize anito-cel for the treatment of patients with relapsed or refractory multiple myeloma (RRMM). Anito-cel is currently being developed in a Phase 2 registrational study and a Phase 3 pivotal study for RRMM. Kite and Arcellx will jointly commercialize the anito-cel asset in the United States, and Kite will commercialize the product outside the United States.
About Gilead Sciences
Gilead Sciences, Inc. is a biopharmaceutical company that has pursued and achieved breakthroughs in medicine for more than three decades, with the goal of creating a healthier world for all people. The company is committed to advancing innovative medicines to prevent and treat life-threatening diseases, including HIV, viral hepatitis, COVID-19, cancer, and inflammation. Gilead operates in more than 35 countries worldwide, with headquarters in Foster City, Calif.
About Kite
Kite, a Gilead Company, is a global biopharmaceutical company based in Santa Monica, California, focused on cell therapy to treat and potentially cure cancer. As the global cell therapy leader, Kite has treated more patients with CAR T-cell therapy than any other company. Kite has the largest in-house cell therapy manufacturing network in the world, spanning process development, vector manufacturing, clinical trial supply and commercial product manufacturing.