-
In ATTAIN-MAINTAIN, orforglipron achieved the primary and all key secondary endpoints for weight maintenance vs. placebo at 52 weeks following weight loss on Wegovy or Zepbound
-
Participants who switched to orforglipron from Wegovy maintained all but 0.9 kg of their previously achieved weight loss on average
-
Lilly has submitted orforglipron to the U.S. Food and Drug Administration for the treatment of obesity
INDIANAPOLIS, Dec. 18, 2025 /PRNewswire/ -- Eli Lilly and Company (NYSE: LLY) today announced positive topline results from the ATTAIN-MAINTAIN trial. The Phase 3 study evaluated orforglipron, an investigational, once-daily oral small molecule glucagon-like peptide-1 (GLP-1) receptor agonist, for weight maintenance over 52 weeks after initial treatment for 72 weeks with the highest tolerated doses of Wegovy (semaglutide) or Zepbound (tirzepatide), in participants from SURMOUNT-5 who were offered the opportunity to be re-randomized to receive orforglipron or placebo. At one year, orforglipron met the primary and all key secondary endpoints compared to placebo, delivering superior weight maintenance as an adjunct to a healthy diet and physical activity, using the efficacy estimand and modified treatment-regimen estimand.1,2
"Obesity is a chronic, progressive disease, and sustaining weight loss remains a significant challenge for many," said Kenneth Custer, Ph.D., executive vice president and president, Lilly Cardiometabolic Health. "ATTAIN‑MAINTAIN showed that orforglipron, a once-daily oral GLP-1, helped people maintain the weight they worked hard to lose. Participants in this study were able to switch directly from the highest tolerated doses of available injectable therapies onto oral doses of orforglipron. If approved for the treatment of obesity, orforglipron could provide a convenient alternative for the millions of individuals living with obesity around the globe to continue their long-term health journey."
In the study, orforglipron met the primary endpoint of superior percent maintenance of body weight reduction compared to placebo, among SURMOUNT-5 participants who previously reached a body weight plateau. In pre-specified analyses at 52 weeks, participants who switched to orforglipron from Wegovy maintained their previously achieved weight loss with an average difference of 0.9 kg, while those who switched to orforglipron from Zepbound maintained their previously achieved weight loss with an average difference of 5.0 kg, using the efficacy estimand. In post-hoc analyses at 24 weeks, the last time point before placebo participants were eligible for orforglipron as rescue therapy, the change in body weight from ATTAIN-MAINTAIN baseline for patients switching to orforglipron from Wegovy was -0.1 kg vs. 9.4 kg for placebo. Likewise, for patients switching to orforglipron from Zepbound, the change from baseline was 2.6 kg vs. 9.1 kg for placebo.
Average Weight at Baseline and ATTAIN-MAINTAIN Results
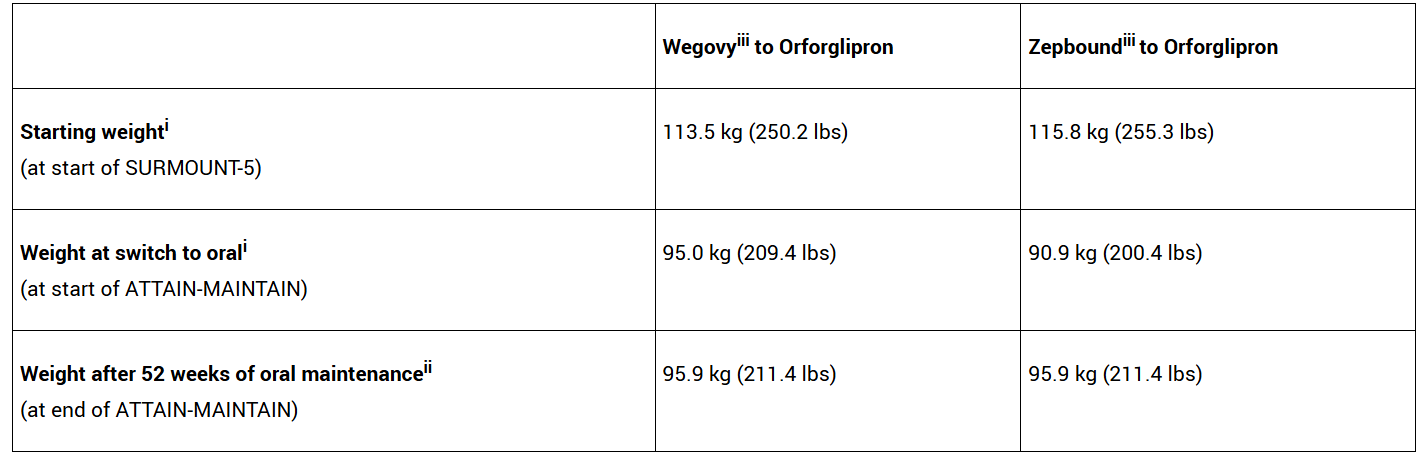
iObserved mean based on efficacy estimand data set
iiMixed Model for Repeated Measures (MMRM) based on efficacy estimand data set
iiiTreatment was at maximum tolerated doses of either 1.7 mg or 2.4 mg (Wegovy) or 10 mg or 15 mg (Zepbound)
The overall safety and tolerability profile of orforglipron in ATTAIN-MAINTAIN was consistent with previous orforglipron Phase 3 studies. The most common adverse events were gastrointestinal-related and generally mild-to-moderate in severity. Discontinuation rates due to adverse events for patients randomized to placebo or orforglipron were 4.8% (orforglipron from Wegovy), 7.6% (placebo from Wegovy), 7.2% (orforglipron from Zepbound) and 6.3% (placebo from Zepbound). No hepatic safety signal was observed.
Detailed results from the ATTAIN-MAINTAIN trial will be presented at a future medical meeting and published in a peer-reviewed journal next year. Lilly has submitted a new drug application to the U.S. Food and Drug Administration (FDA) for orforglipron for the treatment of adults with obesity or overweight. Orforglipron was granted a Commissioner's National Priority Voucher from the U.S. FDA.
About orforglipron
Orforglipron (or-for-GLIP-ron) is an investigational, once-daily small molecule (non-peptide) oral glucagon-like peptide-1 receptor agonist that can be taken any time of the day without restrictions on food and water intake.3 Orforglipron was discovered by Chugai Pharmaceutical Co., Ltd. and licensed by Lilly in 2018. Chugai and Lilly published the preclinical pharmacology data of this molecule together.4 Lilly is running Phase 3 studies on orforglipron for the treatment of type 2 diabetes and for weight management in adults with obesity or overweight with at least one weight-related medical problem. It is also being studied as a potential treatment for obstructive sleep apnea, hypertension, and knee osteoarthritis in adults with obesity, as well as stress urinary incontinence and cardiovascular and renal outcomes.
About ATTAIN-MAINTAIN trial and ATTAIN clinical trial program
ATTAIN-MAINTAIN (NCT06584916) was a 52-week, Phase 3, randomized, double-blind, placebo-controlled trial comparing the efficacy and safety of once-daily orforglipron versus placebo for maintenance of body weight reduction in adults with obesity or overweight with weight-related comorbidities who previously completed the SURMOUNT-5 head-to-head trial. The ATTAIN-MAINTAIN trial randomized 376 participants across the U.S. in a 3:2 ratio to receive either orforglipron maximum tolerated dose (MTD; 24 mg or 36 mg) or placebo, as an adjunct to healthy diet and physical activity.
The primary endpoint was to demonstrate that orforglipron is superior to placebo in the maintenance of body weight reduction in participants who achieved plateau with either Zepbound or Wegovy in the SURMOUNT-5 trial. Weight plateau was defined as <5% body weight change between weeks 60 and 72 in SURMOUNT-5. In ATTAIN-MAINTAIN, participants were randomized to a 12 mg dose of once-daily, oral orforglipron (or matching placebo) which was increased every 4 weeks until the randomized maintenance dose of 36 mg or MTD (24 mg or 36 mg) was reached. All participants who regained 50% or more of their body weight from SURMOUNT-5 were treated with rescue orforglipron MTD therapy in this novel, patient-centric approach to clinical trial design.
The ATTAIN Phase 3 global clinical development program for orforglipron has enrolled more than 4,500 people with obesity or overweight across two global registrational trials.
About SURMOUNT-5
SURMOUNT-5 (NCT05822830) was a 72-week, multi-center, randomized, open-label, Phase 3b trial evaluating the efficacy and safety of Zepbound (tirzepatide) compared with Wegovy (semaglutide) in adults with obesity, or overweight with at least one of the following comorbidities: hypertension, dyslipidemia, obstructive sleep apnea (OSA) or cardiovascular disease, who did not have diabetes. Participants in both treatment groups received counseling on a reduced-calorie diet and increased physical activity. The trial randomized 751 participants across the U.S. and Puerto Rico in a 1:1 ratio to receive maximum tolerated dose of Zepbound (10 mg or 15 mg) or Wegovy (1.7 mg or 2.4 mg). With Zepbound, 89.3% received at least one dose of the 15 mg dose and with Wegovy 92.8% received at least one dose of the 2.4 mg dose. The primary objective of the study was to demonstrate Zepbound's superiority in percentage change from baseline in body weight at 72 weeks compared to Wegovy. In the SURMOUNT-5 trial, participants treated with Zepbound achieved an average weight reduction of 20.2% compared to 13.7% with Wegovy at 72 weeks.
About tirzepatide
Tirzepatide is a once-weekly dual GIP (glucose-dependent insulinotropic polypeptide) receptor and GLP-1 (glucagon-like peptide-1) receptor agonist. Tirzepatide is a single molecule that activates the body's receptors for GIP and GLP-1, which are natural incretin hormones. Both GIP and GLP-1 receptors are found in areas of the human brain important for appetite regulation. Tirzepatide decreases calorie intake, and the effects are likely mediated by affecting appetite. Studies of tirzepatide in chronic kidney disease (CKD) and in morbidity/mortality in obesity (MMO) are ongoing.
Tirzepatide has been approved by the U.S. FDA as Mounjaro for adults with type 2 diabetes to improve glycemic control, and as Zepbound for adults with obesity, or some adults who are overweight and also have at least one weight-related medical problem, to lose weight and keep it off. Additionally, Zepbound is FDA-approved to treat adults with moderate-to-severe obstructive sleep apnea and obesity. Tirzepatide is also approved as Mounjaro in some countries outside the U.S. for adults with type 2 diabetes, obesity or those who are overweight who also have a weight-related comorbid condition. Both Mounjaro and Zepbound should be used in combination with diet and exercise.
About Lilly
Lilly is a medicine company turning science into healing to make life better for people around the world. We've been pioneering life-changing discoveries for nearly 150 years, and today our medicines help tens of millions of people across the globe. Harnessing the power of biotechnology, chemistry and genetic medicine, our scientists are urgently advancing new discoveries to solve some of the world's most significant health challenges: redefining diabetes care; treating obesity and curtailing its most devastating long-term effects; advancing the fight against Alzheimer's disease; providing solutions to some of the most debilitating immune system disorders; and transforming the most difficult-to-treat cancers into manageable diseases. With each step toward a healthier world, we're motivated by one thing: making life better for millions more people. That includes delivering innovative clinical trials that reflect the diversity of our world and working to ensure our medicines are accessible and affordable.
Endnotes and References
-
The efficacy estimand represents efficacy had all randomized participants remained on study intervention (with possible dose interruptions and/or dose modifications) for 52 weeks without initiating prohibited weight management treatments, and assuming participants who took rescue orforglipron would not have received any additional improvement from their randomized study treatment.
-
The modified treatment-regimen estimand represents the estimated average treatment effect regardless of adherence to study intervention or initiation of prohibited weight management treatments, and assuming participants who took rescue orforglipron would not have received any additional improvement from their randomized study treatment.
-
Ma X, Liu R, Pratt EJ, Benson CT, Bhattachar SN, Sloop KW. Effect of Food Consumption on the Pharmacokinetics, Safety, and Tolerability of Once-Daily Orally Administered Orforglipron (LY3502970), a Non-peptide GLP-1 Receptor Agonist. Diabetes Ther. 2024 Apr;15(4):819-832. doi: 10.1007/s13300-024-01554-1. Epub 2024 Feb 24. PMID: 38402332; PMCID: PMC10951152.
-
T. Kawai, B. Sun, H. Yoshino, D. Feng, Y. Suzuki, M. Fukazawa, S. Nagao, D.B. Wainscott, A.D. Showalter, B.A. Droz, T.S. Kobilka,
-
M.P. Coghlan, F.S. Willard, Y. Kawabe, B.K. Kobilka, & K.W. Sloop
-
, Structural basis for GLP-1 receptor activation by LY3502970, an orally active nonpeptide agonist, Proc. Natl. Acad. Sci. U.S.A. 117 (47) 29959-29967, https://doi.org/10.1073/pnas.2014879117 (2020).