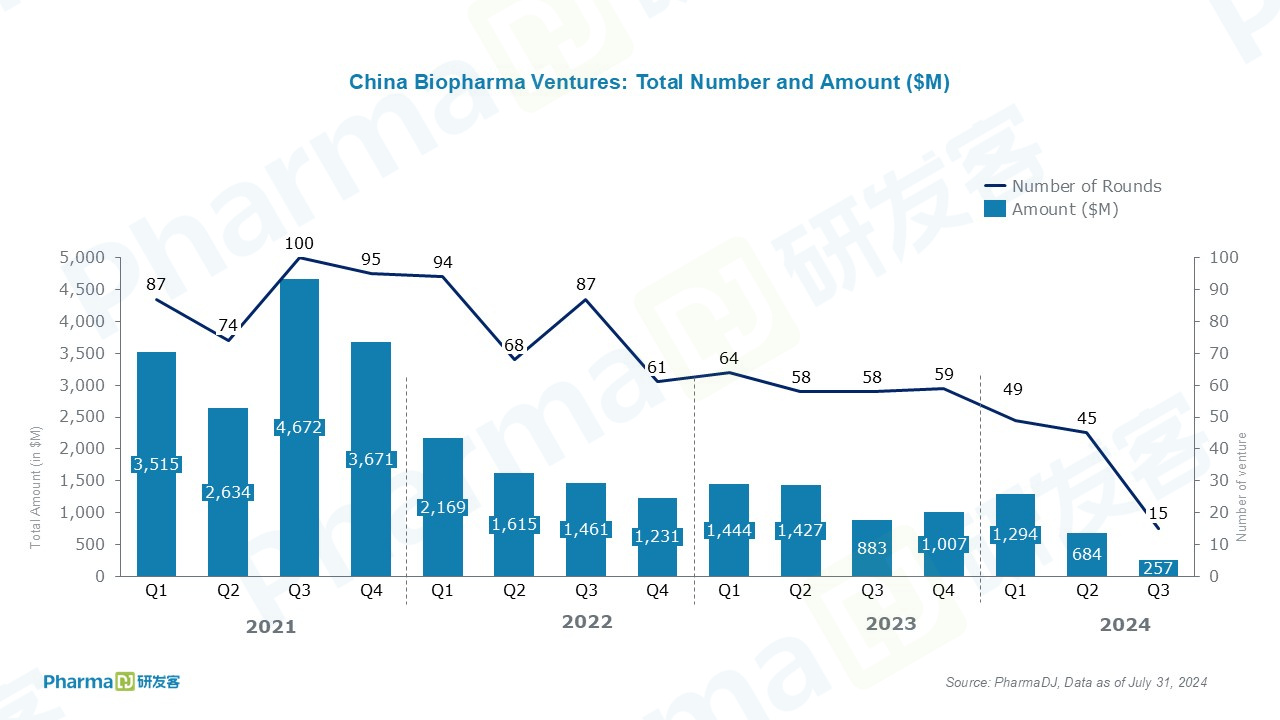
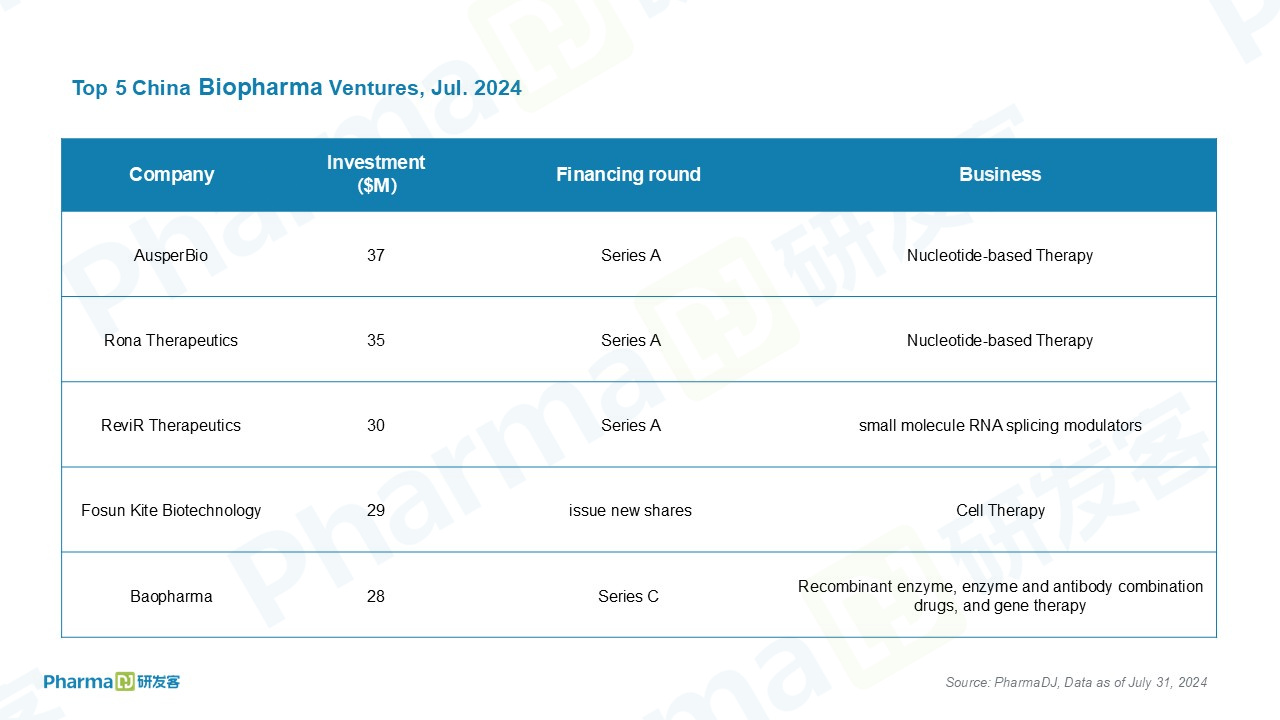
Based on data gathered by PharmaDJ, 15 privately held Chinese drugmakers obtained financing in 2024. 12 disclosed funding worth a total of $257 million.
The leading funding recipient is AusperBio, a clinical-stage biotechnology company dedicated to advancing targeted oligonucleotide therapies. The company has closed a Series A financing round of $37 million led by existing investor, InnoPinnacle Fund.
The proceeds from the financing will further support the clinical development of AHB-137, AusperBio’s lead product candidate. Additionally, the funds will advance its proprietary Med-Oligo technology platform and its product pipelines.
AHB-137 is a novel dual-mechanism unconjugated antisense oligonucleotide (ASO) developed within Med-Oligo platform, was designed to treat chronic hepatitis B for a functional cure. It is presently undergoing a Phase 1b trial across multiple international study sites and a concurrent Phase 2 trial in China.
ADC-focused ProfoundBio raised $112 million in oversubscribed Series B round to push several candidates forward. The Seattle-based biotech has three clinical-stage candidates in the portfolio and has a R&D office in Suzhou, China.
There were no initial public offerings for Chinese drug developers in July.
No new Chinese biotech M&A deal announced the month.
11 licensing deals for Chinese drugmakers were disclosed, worth a total of $3.9 billion, including $39 million in upfront payments.
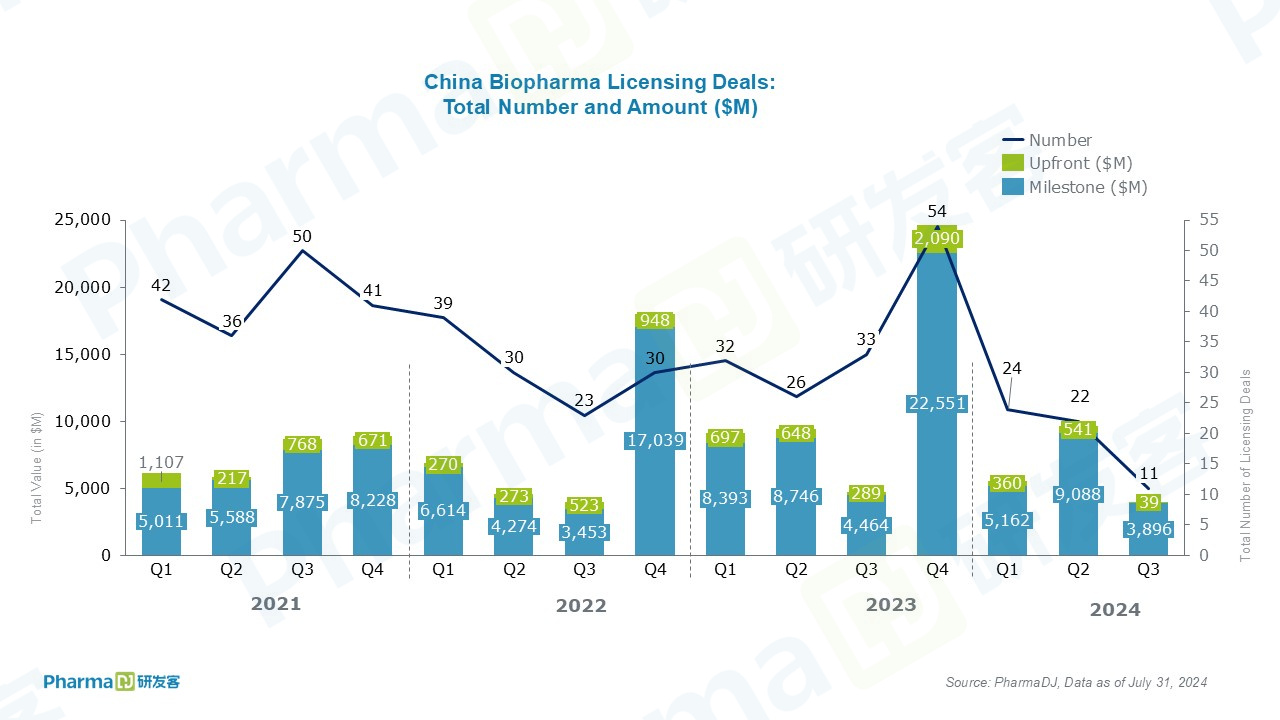
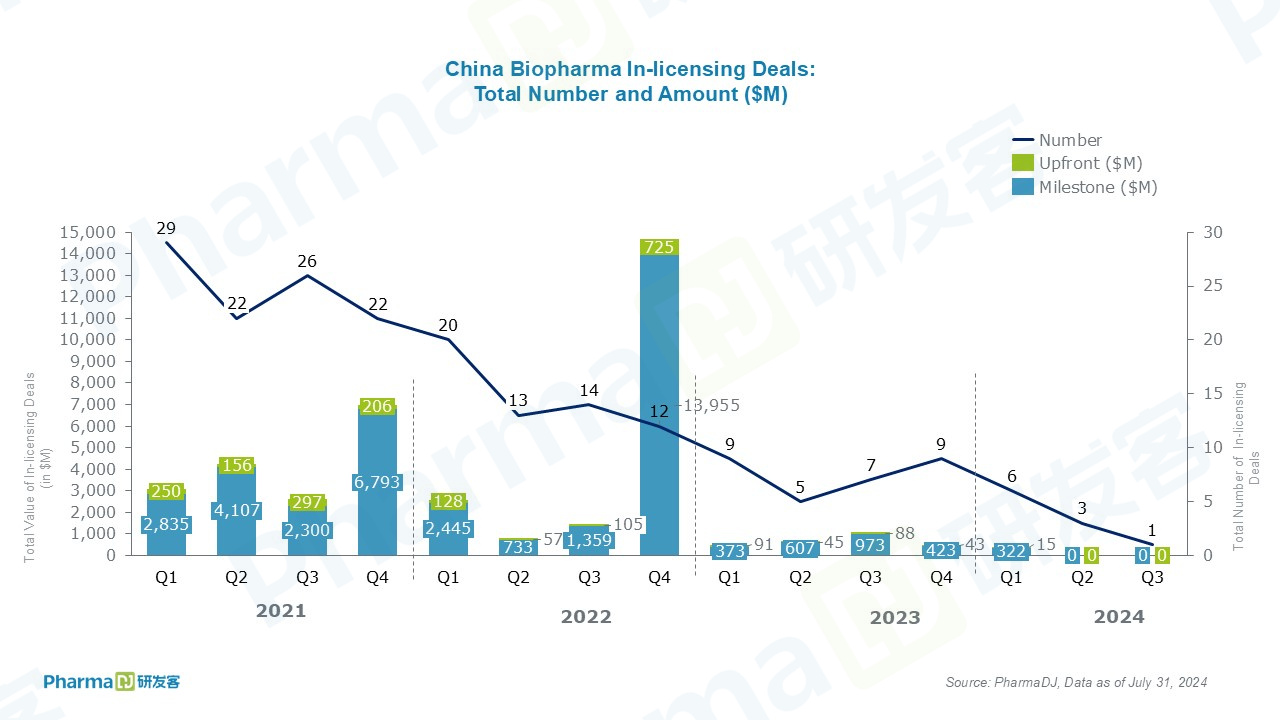
In-licensing deals are only one, and no financial terms have been disclosed.
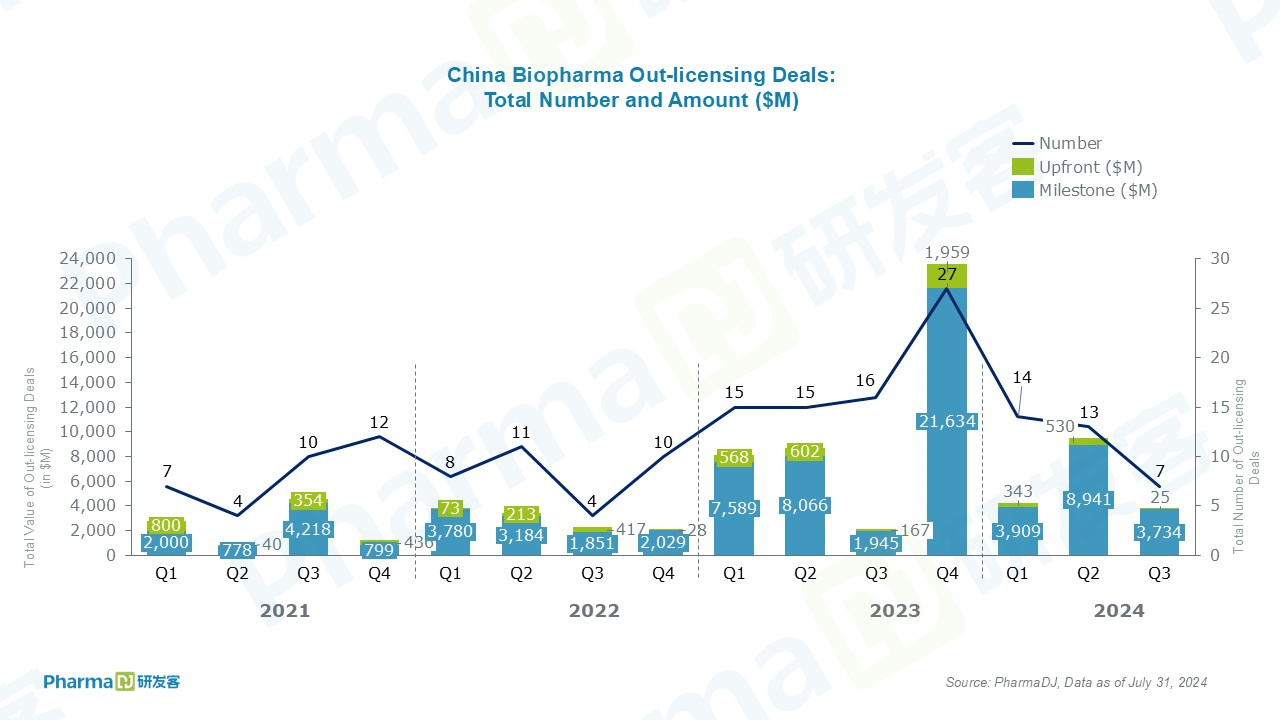
Out-licensing deals are also seven, worth a total of $3.8 billion, including $25 million in upfront payments..
Other three licensing deals are reached between Chinese drugmakers
Nanjing-based 3D printing company Triastek signed a deal with BioNTech to create oral bioavailable RNA therapeutics that optimise delivery across the gastrointestinal (GI) mucosa and minimise degradation in the GI tract so the compound can reach a portion of the GI system where absorption can be maximised.
Triastek will receive $10 million upfront and is eligible for development, regulatory, and commercial milestones worth potentially more than $1.2 billion, plus tiered royalties.
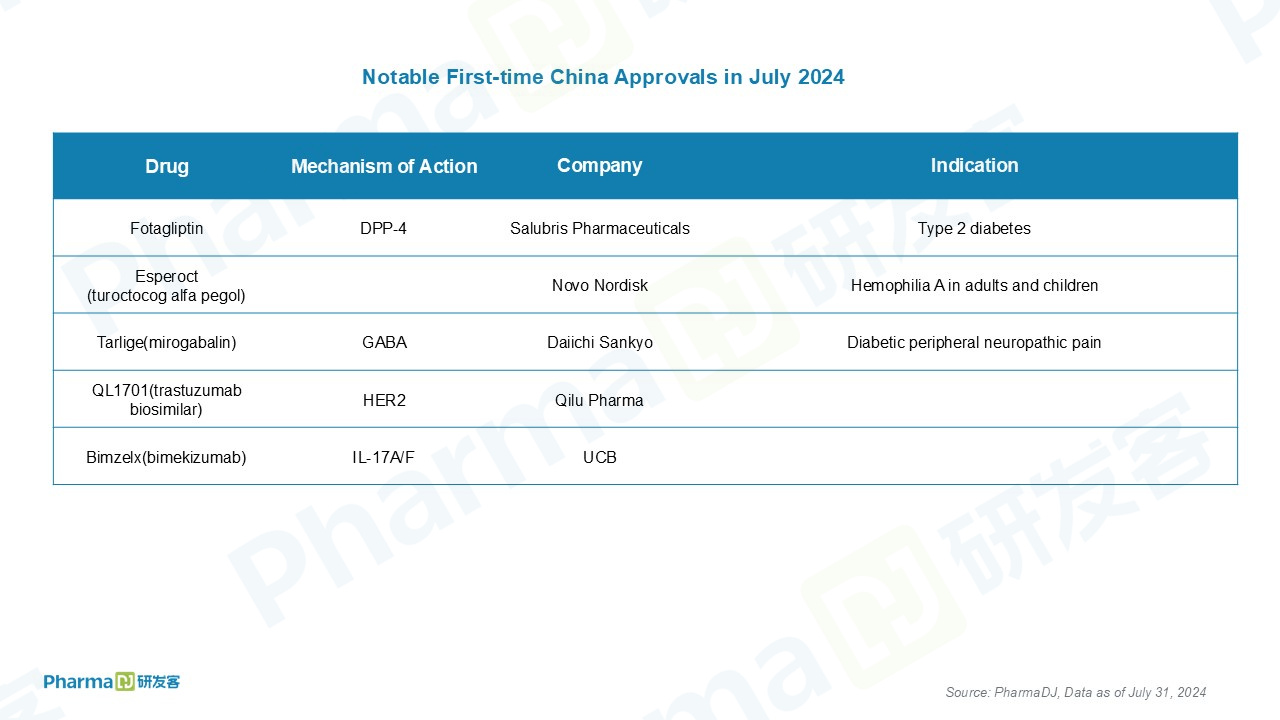
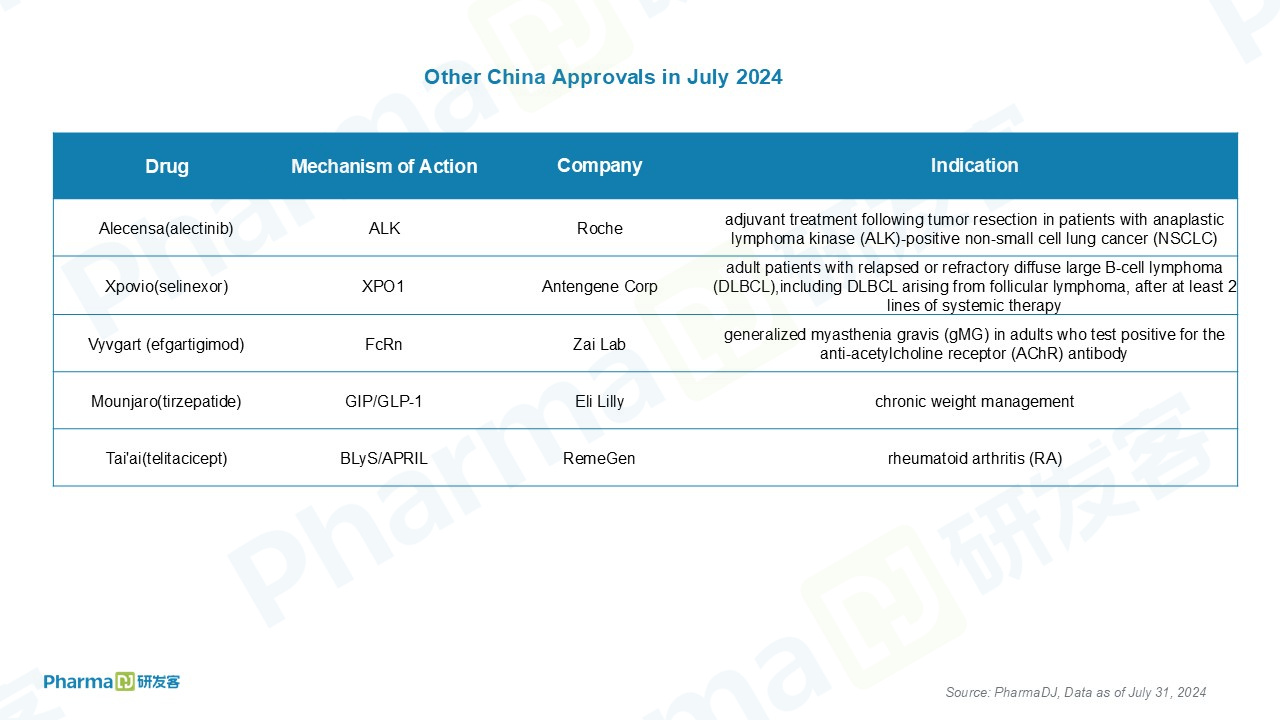
The notable drug approvals in China are as below.