By Yingzi Shi, Minhua Chu
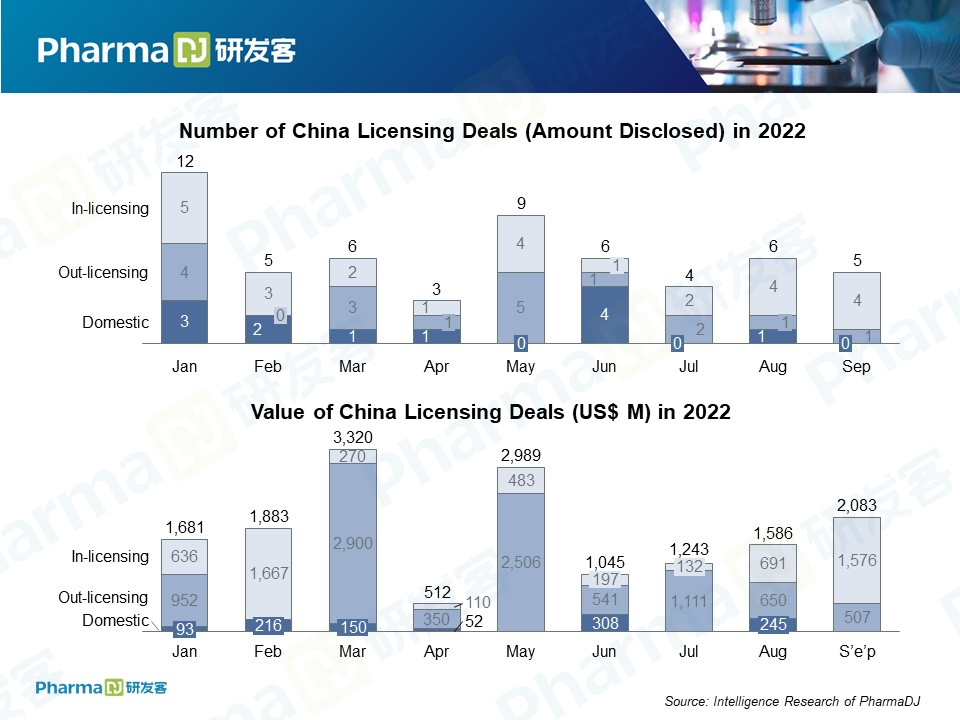
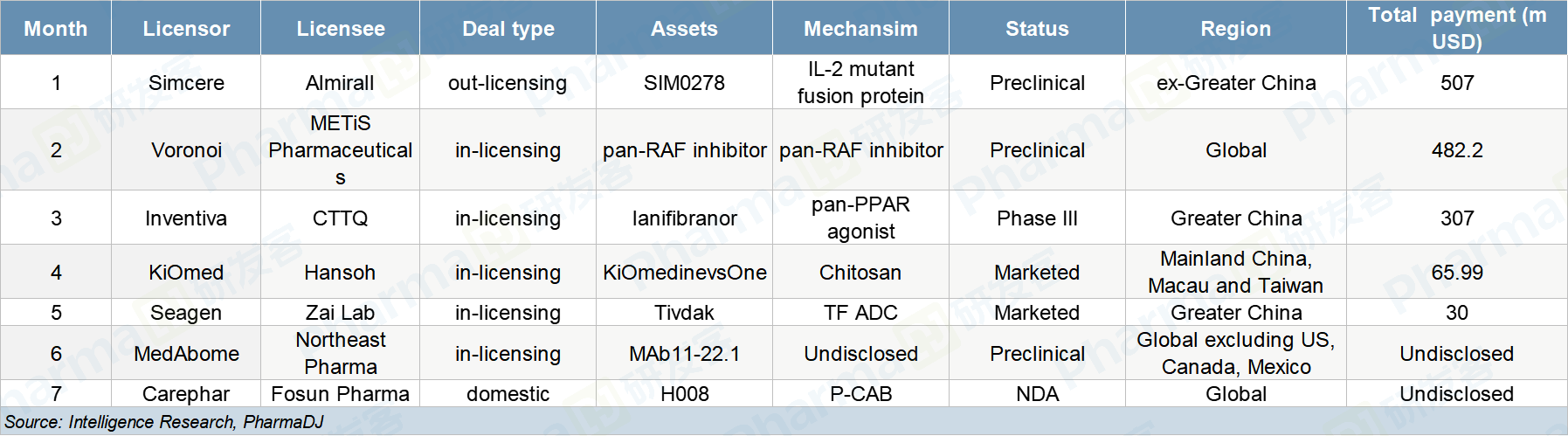
September was a slow month in pharmaceutical licensing deals, only seven. But, despite low volume, the five deals that were disclosed amounted to a total of $2.083 billion, ranking September third below March and May in terms of deal value.
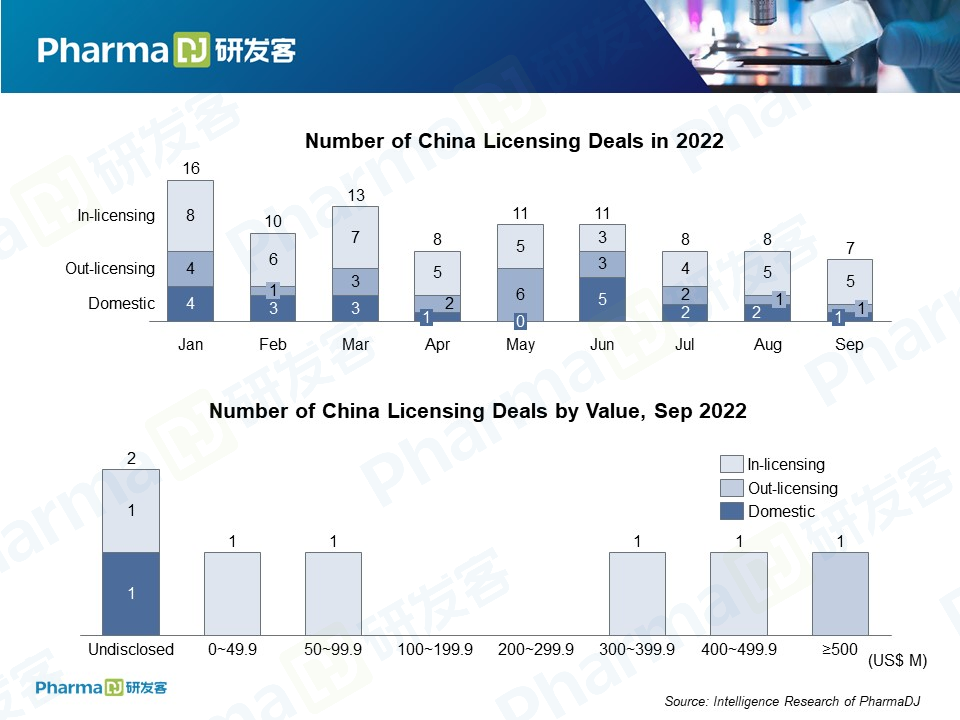
Five of them were in-licensing deals with overseas companies. One was an out-licensing deal, while the other was inked between two domestic drugmakers.
The biggest deal of the month was between Simcere Pharmaceutical, one of China’s leading drugmakers, and Spain-based Almirall. Simcere sold its experimental IL-2 mutant fusion protein SIM0278 to Almirall. For an upfront payment $15 million, up to $492 million in milestones and low double-digit tiered royalties based on future sales, Almirall will obtain exclusive rights to SIM0278 outside of Greater China.
In-licensing, however, took up the lion’s share of September’s deals, with a total value of $1.576 billion — double that of August and the second highest monthly figure this year. This could be a sign that in-licensing deals a resurgent as we enter Q4.
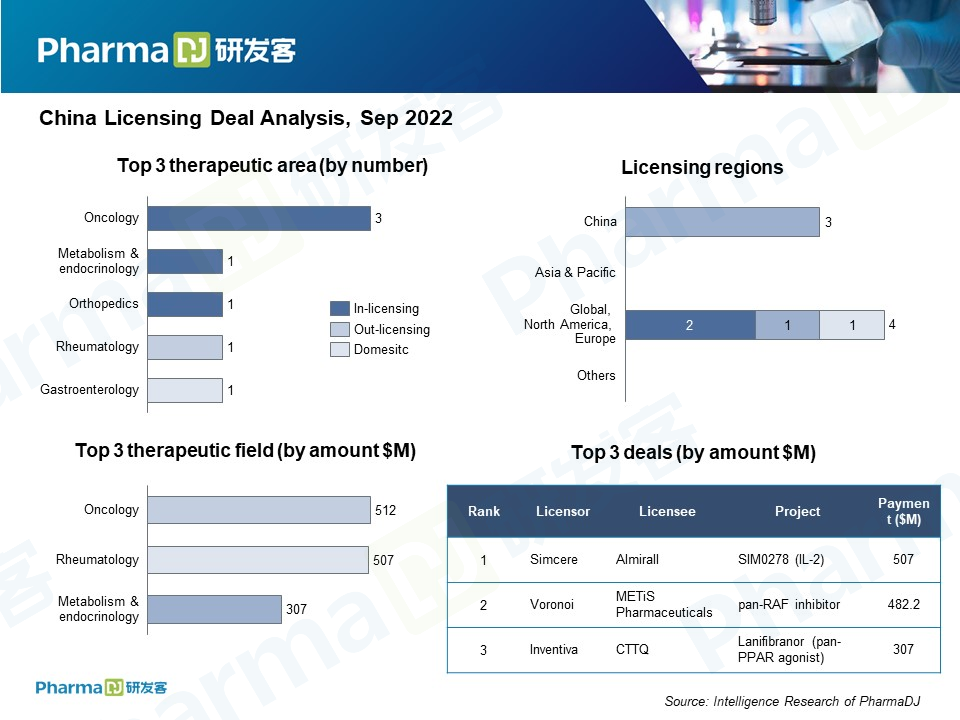
METis Pharmaceuticals buys a nucleic acid drug from Voronoi
Big drugmakers like Simcere, CTTQ, and Hansoh dominated the deal space in September with one glaring exception, METiS Pharmaceuticals. The Chinese startup announced that it would be bringing a pan-RAF inhibitor into China from South Korean drugmaker Voronoi. METiS will pay an initial $1.7 million and will also make milestone payments of up to $480.5 million, making it the month’s second largest deal.
Founded in 2020 and incubated by XtalPi, an AI-powered drug discovery service platform, METiS is an innovative AI-driven drug delivery and formulation development company. It joined the Roche Accelerator in May 2022.
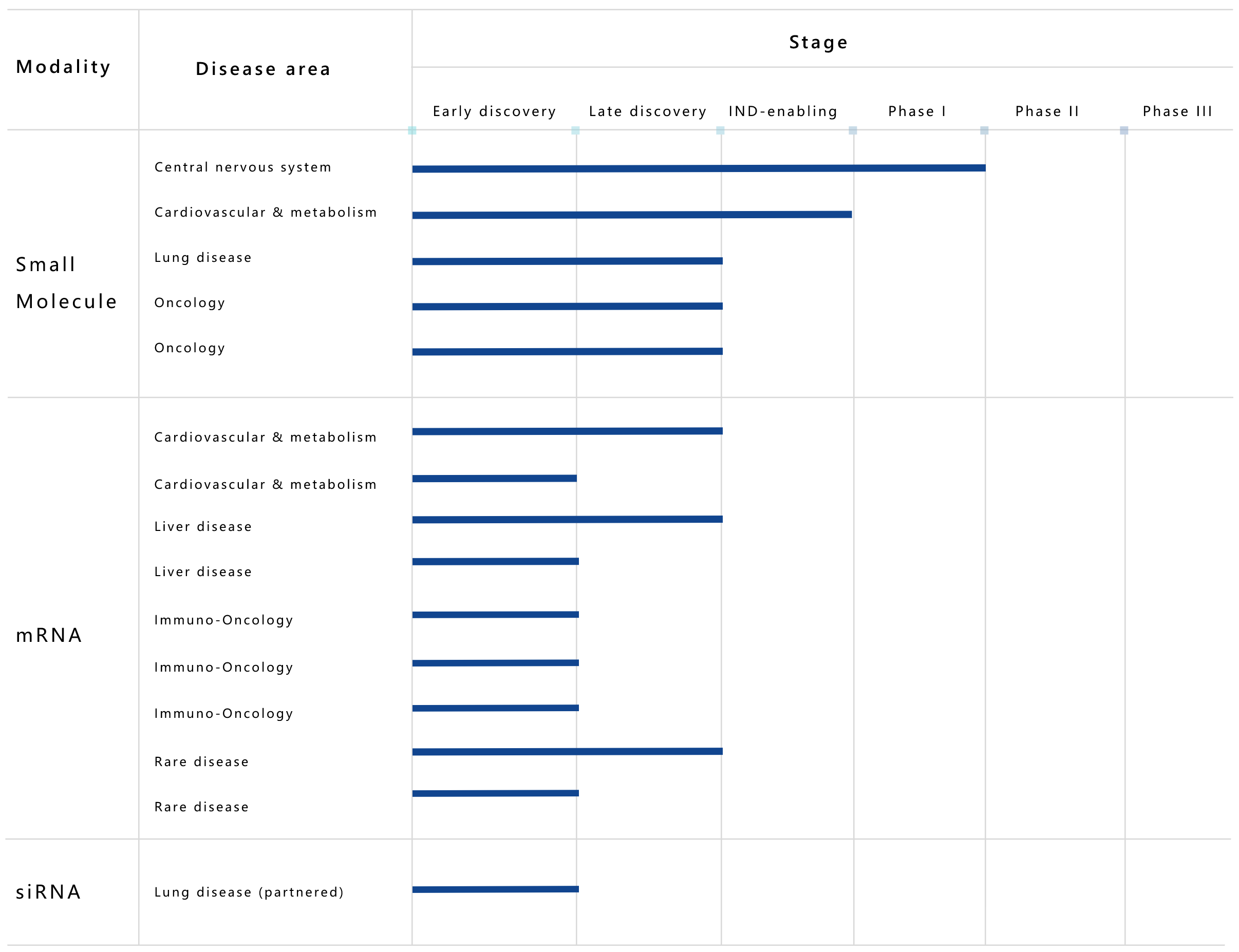
Its pipelines include new formulations of marketed products and nucleic acid drugs. Its leading product, MTS004, a modified drug for pseudobulbar affect (PBA), has already entered Phase 1 studies. Its nucleic acid drug pipeline includes mRNA and siRNA therapies for cardiovascular, metabolic, liver and rare diseases.
METiS just completed two consecutive financing rounds totaling $150 million in the first half of this year. Investors were optimistic about the company’s nucleic acid drug platform. This latest deal signals that nucleic acid drugs will be the company’s focus in the future. It also provides China’s mRNA vaccine and drugmakers for future development.
Pharma DJ’s research shows that drugmakers developing nucleic acid products were involved in 17 financing deals at a total value of $600 million in the January-September period of this year? In other words, companies in this space have plenty of cash flow.
However, most of these companies began developing nucleic acid products with the goal of producing an mRNA vaccine for COVID-19. Achieving that goal appears increasingly less feasible. No mRNA vaccine has received market approval in China yet. Sales in Western countries are slumping too. Companies are therefore pivoting from disease prevention to disease treatment, especially tumors.
However, it’s difficult to develop new products quickly. Introducing innovative candidates from overseas start-ups may prove to be a sound business decision for Chinese companies developing mRNA-based treatments.
Zai Lab enters the ADC space
Finally, Zai Lab and Seagen inked the deal with the month’s highest upfront payment, $30 million. Milestone payments were not disclosed. Through the deal, Zai Lab obtained Seagen’s ADC drug tisotumab vedotin. This is new territory for Zai Lab. Like antibodies, ADCs are another emergent field in China. Earlier this year, the NMPA’s CDE released a series of standardization guidelines for the development of ADC drugs.
Edited by Justin Fischer